Abstract
Polymethyl methacrylate (PMMA) is a polymer with excellent properties for water remediation. Understanding the molecular interactions between pharmaceuticals, such as β-blockers, and PMMA is essential for optimizing purification technologies. Atomistic calculations provide a detailed understanding of the interaction between molecules without the need for expensive equipment. This study presents a computational analysis of how PMMA interacts with salbutamol and atenolol. Geometrical optimizations were performed using semiempirical and density functional theory (DFT) calculations. To identify interactions between PMMA and pharmaceuticals, we employed the reduced density gradient (RDG) approach, providing insight into intramolecular noncovalent interactions between PMMA’s atoms and pharmaceuticals.
1. Introduction
Water stress is a significant issue globally, with many regions experiencing water scarcity. The main challenge in achieving effective wastewater treatment is maximizing the overall efficiency of treatment systems [1]. As a result, there has been growing interest in developing functional materials, which has led to the emergence and application of advanced polymers. Advanced polymers have demonstrated practical applications in a variety of fields, including renewable energy, medical diagnostics, water treatment, pollution control, environmental protection, and food safety. Their increasing use can be attributed to their high sensitivity, versatility, specificity, and capability for real-time analysis. Polymers are significant in environmental applications, especially in water treatment systems [2].
Research has revealed a wide variety of pharmaceuticals, steroid hormones, and other non-regulated organic compounds present in the environment. These contaminants of emerging concern have been found in the effluents from wastewater treatment plants, in surface water, and even in drinking water systems [3]. Among these, certain pharmaceuticals, such as β-blockers, are frequently detected in water sources. These substances pose considerable risks due to their persistence and bioactivity. The presence of these emerging contaminants has prompted numerous studies aimed at better understanding their fate, transport, and removal [4].
Salbutamol, also referred to as albuterol, is the most widely used β2-agonist for the treatment of bronchial asthma. The drug is excreted in urine as a mix of unchanged salbutamol and its conjugated metabolite. It has been detected in several European countries, specifically in rivers and sewage treatment plants, with concentrations ranging from 1.14 to 471 ng/dm3 [5].
Atenolol, a selective β1 receptor antagonist, has been primarily used for the treatment of cardiovascular diseases for over thirty years. Due to its widespread use and limited metabolism in humans, atenolol is commonly found in sewage effluents and surface water. Atenolol has been detected in surface and groundwater, with concentrations ranging from 5 to 560 ng/dm3 [6].
Polymethyl methacrylate (PMMA) is a polymer with excellent mechanical, optical, and electrical properties. It is also economical, non-toxic, and insoluble in water, making it an ideal material for water remediation. Understanding the molecular interactions between pharmaceutical pollutants and PMMA is essential for optimizing filtration and purification technologies [7].
The computational studies of advanced materials primarily focus on their atomic interactions to understand the unique properties derived from their structures for the development of new technologies. Atomic interactions govern the behavior of materials at the macroscopic level, and simulations help to provide a clearer understanding of the materials’ properties. Atomistic calculations offer additional insights into interactions between molecules, therefore supplementing experimental findings [8].
This study presents a computational analysis of how PMMA interacts with salbutamol and atenolol. Geometrical optimizations were performed using semiempirical and density functional theory (DFT) calculations. To identify significant interactions between PMMA and pharmaceuticals, we employed the reduced density gradient (RDG) approach, providing insight into intramolecular noncovalent interactions between PMMA’s atoms and pharmaceutical molecules. These findings illuminate the fundamental mechanisms of PMMA interactions with pharmaceuticals, offering valuable insights for its use in the environmental remediation of pharmaceutical pollutants.
2. Computational Details
Geometrical optimizations of all the structures were conducted using the GFN2 method developed by Prof. Stefan Grimme and coworkers [9,10,11,12,13]. Binding energies were calculated using the same level of theory. Optimized geometries were further used to perform single-point energy calculations using the DFT method, employing the M06-2X [14,15,16,17] density functional and 6-31G(d,p) basis set.
GFN2 calculations have been performed using the xtb 6.7.1. code [10,11], as implemented in the atomistica.online molecular modeling platform [18,19], freely available at https://atomistica.online. In particular, the Online xtb tool of the atomistica.online was used for these purposes. DFT calculations were performed with the ORCA modeling package [20,21,22,23,24,25,26], a program created by Prof. Frank Nesse and coworkers. RDG scatter plots and cub files for obtaining RDG surfaces were obtained using the Online RDG tool of the atomistica.online, which uses the Multiwfn program [27,28,29,30] in the background, a program created and maintained by Prof. Tian Lu. RDG surfaces were obtained using the VMD program [31,32,33].
3. Results and Discussion
The structure of PMMA, presented as a pentamer, along with the structures of the tested pharmaceuticals, are shown in Figure 1. To investigate the interaction between PMMA and pharmaceuticals, two systems (PMMA/salbutamol and PMMA/atenolol) were geometrically optimized using the GFN2 method. The GFN2 method is significantly faster than DFT methods due to its semiempirical nature. Despite some approximations, GFN2 effectively provides reliable structural properties of intermolecular complexes [11]. Four conformations for each system were generated and optimized.
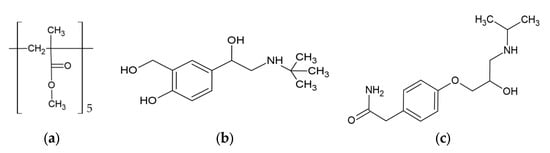
Figure 1.
The structures of (a) PMMA, (b) salbutamol, and (c) atenolol.
Binding energy values are crucial for understanding how molecules adsorb and desorb on material surfaces. They provide insights into interactions between molecular structures. Based on the calculated binding energies for all the selected conformations, we chose those with the most potent binding energies for further analysis. The calculated binding energies for selected conformations of the PMMA/salbutamol and PMMA/atenolol systems are presented in Table 1. The GFN2 calculations for the PMMA/salbutamol and PMMA/atenolol systems show that the binding energy in the case of salbutamol is higher than that of atenolol. This indicates that PMMA might have a stronger binding affinity for salbutamol.

Table 1.
Binding energies between PMMA and pharmaceuticals.
To determine which atoms contribute to the interaction between PMMA and the pharmaceuticals under consideration, we identified and quantified the noncovalent interactions between these molecules. We detected these noncovalent interactions by analyzing the electron density among all the atoms, mainly by applying the RDG approach for a deeper understanding of these interactions.
An overview of the noncovalent interactions between the observed pharmaceuticals and the polymer can be obtained by analyzing the RDG value in relation to (λ2)ρ, as shown in Figure 2 The RDG scatter plots help to identify interaction types based on sign(λ2)ρ. Blue dots (λ2 < 0, ρ > 0) indicate strong, attractive interactions, suggesting hydrogen bond formation. Green dots (λ2 and ρ ≈ 0) represent weak van der Waals interactions, while red dots (λ2 > 0, ρ > 0) indicate strong repulsion.
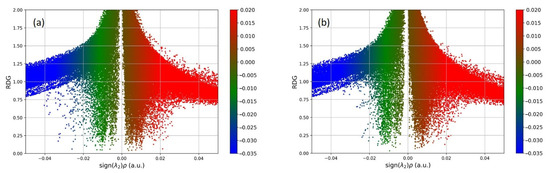
Figure 2.
RDG scatter plots of (a) PMMA/salbutamol and (b) PMMA/atenolol systems.
A more significant number of blue dots are observed in the PMMA/salbutamol system (Figure 2a) compared to the PMMA/atenolol system, indicating stronger binding. The red dots are represented approximately equally in both systems. A more detailed view of how pharmaceuticals bind to the PMMA polymer can be seen in Figure 3.
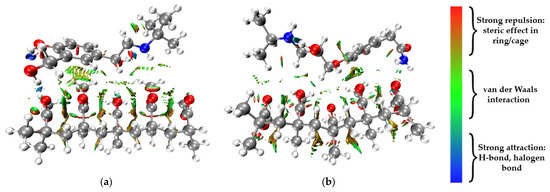
Figure 3.
Graphical illustration of the RDG surface and intramolecular noncovalent interactions of (a) PMMA/salbutamol and (b) PMMA/atenolol systems.
Figure 3 illustrates the RDG surface for the interaction between PMMA and pharmaceuticals. The RDG analysis reveals distinct and intense surfaces in the PMMA/salbutamol system, specifically between the hydrogen atom of the hydroxyl group in the salbutamol molecule and the oxygen atom in the PMMA polymer chain. This interaction is represented by a blue color, indicating relatively strong noncovalent interactions characterized by hydrogen bonding. Additionally, strong interactions are evident between the benzene ring of the salbutamol molecule and the hydrogen atoms of the methyl acetate group in the PMMA chain. These RDG surfaces primarily appear green, suggesting weak van der Waals interactions. However, they still contribute to the overall noncovalent binding strength between salbutamol and PMMA.
The RDG analysis of the PMMA/atenolol system reveals an interaction between the benzene ring of the atenolol molecule and the hydrogen atoms of the methyl acetate group in the PMMA chain (Figure 3). This interaction is represented by a yellow-to-green color gradient, indicating weak van der Waals interactions. These results are in accordance with the obtained binding energies.
4. Conclusions
Atomistic calculations provide valuable insights into molecular interactions, which support the development of advanced PMMA-based materials designed for removing pharmaceuticals from water. We conducted a detailed investigation into the interactions between the PMMA polymer and two pharmaceuticals: salbutamol and atenolol. Our computational study employed semiempirical and DFT calculations. Initially, the PMMA/salbutamol and PMMA/atenolol systems were geometrically optimized using the GFN2 method. Following this, DFT calculations were performed to identify and quantify the noncovalent interactions between PMMA and the selected pharmaceuticals. The binding energy calculations revealed that salbutamol binds more strongly to the PMMA chain than atenolol does. Furthermore, we found that the strongest noncovalent interactions characterize the PMMA/salbutamol system. These findings illuminate the fundamental mechanisms of PMMA interactions with pharmaceuticals, offering valuable insights for its use in the environmental remediation of pharmaceutical pollutants.
Author Contributions
Conceptualization, A.B., S.J.A. and S.A.; methodology, S.J.A. and S.A.; software, S.J.A. and S.A.; validation, S.P., S.J.A. and S.A.; formal analysis, A.B.; investigation, A.B., D.K., S.J.A. and S.A.; resources, S.P., S.J.A. and S.A.; data curation, A.B., D.K. and S.A.; writing—original draft preparation, S.P., A.B., D.K., S.J.A. and S.A.; writing—review and editing, A.B., S.J.A. and S.A.; visualization, A.B. and D.K.; supervision, S.P., S.J.A. and S.A.; project administration, S.P., S.J.A. and S.A.; funding acquisition, S.P., S.J.A. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Scientific and Technological Development and Higher Education of the Republic of Srpska (19.032/961-90/24) and the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (451-03-66/2024-03/200125, 451-03-65/2024-03/200125, and 451-03-66/2024-03/200358).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
Acknowledgments
A.B., S.J.A. and S.A. are grateful to the AIDASCO (https://aidasco.org/ accessed on 12 March 2025), who supported the research by providing some of the computer resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The Role of Wastewater Treatment in Achieving Sustainable Development Goals (SDGs) and Sustainability Guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- EL-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gitungo, S.; Dyksen, J.E.; Raczko, R.F.; Axe, L. Indicator Compounds Representative of Contaminants of Emerging Concern (CECs) Found in the Water Cycle in the United States. Int. J. Environ. Res. Public Health 2021, 18, 1288. [Google Scholar] [CrossRef]
- Love, D.; Slovisky, M.; Costa, K.A.; Megarani, D.; Mehdi, Q.; Colombo, V.; Ivantsova, E.; Subramaniam, K.; Bowden, J.A.; Bisesi, J.H., Jr.; et al. Toxicity Risks Associated with the Beta-Blocker Metoprolol in Marine and Freshwater Organisms: A Review: Toxicity of Metoprolol. Environ. Toxicol. Chem. 2024, 43, 2530–2544. [Google Scholar] [CrossRef]
- Zhou, L.; Sleiman, M.; Ferronato, C.; Chovelon, J.-M.; de Sainte-Claire, P.; Richard, C. Sulfate Radical Induced Degradation of Β2-Adrenoceptor Agonists Salbutamol and Terbutaline: Phenoxyl Radical Dependent Mechanisms. Water Res. 2017, 123, 715–723. [Google Scholar] [CrossRef]
- Quaresma, A.V.; Sousa, B.A.; Silva, K.T.S.; Silva, S.Q.; Werle, A.A.; Afonso, R.J.C.F. Oxidative Treatments for Atenolol Removal in Water: Elucidation by Mass Spectrometry and Toxicity Evaluation of Degradation Products. Rapid Commun. Mass Spectrom. 2019, 33, 303–313. [Google Scholar] [CrossRef]
- Krunić, D.; Armaković, S.; Bilić, A.; Armaković, S.J.; Gavanski, L. Understanding the Interactions between PMMA Polymer and Common Industrial Gasses: A Computational xTB, DFT, SAPT and MD Study. Mol. Phys. 2025, e2456108. [Google Scholar] [CrossRef]
- Singh, V.; Patra, S.; Arul Murugan, N.; Toncu, D.-C.; Tiwari, A. Recent Trends in Computational Tools and Data-Driven Modeling for Advanced Materials. Mater. Adv. 2022, 3, 4069–4087. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-Binding Quantum Chemistry Methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Pracht, P.; Caldeweyher, E.; Ehlert, S.; Grimme, S. A Robust Non-Self-Consistent Tight-Binding Quantum Chemistry Method for large Molecules. ChmRxiv, 2019. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All Spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Valero, R.; Costa, R.; de P., R. Moreira, I.; Truhlar, D.G.; Illas, F. Performance of the M06 Family of Exchange-Correlation Functionals for Predicting Magnetic Coupling in Organic and Inorganic Molecules. J. Chem. Phys. 2008, 128, 114103. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpète, E.A.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D.G. On the Performances of the M06 Family of Density Functionals for Electronic Excitation Energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Atomistica.Online—Web Application for Generating Input Files for ORCA Molecular Modelling Package Made with the Anvil Platform. Mol. Simul. 2023, 49, 117–123. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Online and Desktop Graphical User Interfaces for Xtb Programme from Atomistica.Online Platform. Mol. Simul. 2024, 50, 560–570. [Google Scholar] [CrossRef]
- Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An Improved Chain of Spheres for Exchange Algorithm. J. Chem. Phys. 2021, 155, 104109. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, L224108. [Google Scholar] [CrossRef]
- Neese, F. The SHARK Integral Generation and Digestion System. J. Comp. Chem. 2022, 44, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. WIRES Comput. Molec. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. Approximate Second-Order SCF Convergence for Spin Unrestricted Wavefunctions. Chem. Phys. Lett. 2000, 325, 93–98. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 5.0. WIRES Comput. Molec. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIRES Comput. Molec. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Van Der Waals Potential: An Important Complement to Molecular Electrostatic Potential in Studying Intermolecular Interactions. J. Mol. Model. 2020, 26, 315. [Google Scholar] [CrossRef]
- Lu, T.; Manzetti, S. Wavefunction and Reactivity Study of Benzo[a]Pyrene Diol Epoxide and Its Enantiomeric Forms. Struct. Chem. 2014, 25, 1521–1533. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Eargle, J.; Wright, D.; Luthey-Schulten, Z. Multiple Alignment of Protein Structures and Sequences for VMD. Bioinformatics 2006, 22, 504–506. [Google Scholar] [CrossRef]
- Stone, J.; Gullingsrud, J.; Grayson, P.; Schulten, K. A System for Interactive Molecular Dynamics Simulation. In Proceedings of the 2001 ACM Symposium on Interactive 3D Graphics; Hughes, J.F., Séquin, C.H., Eds.; ACM SIGGRAPH: New York, NY, USA, 2001; pp. 191–194. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).