Abstract
A simple urine phantom was developed to replicate the ultraviolet–visible (UV–Vis) spectrum of healthy human urine. Made from four safe and widely available ingredients, it addresses the challenges of biological samples, including safety risks and storage issues. The spectroscopic analysis results confirmed strong similarity to natural urine, making the phantom appropriate for testing spectroscopic methods, calibrating optical devices, and evaluating diagnostic sensors. It can also serve as a starting point for advanced phantoms tailored to specific patient needs or diseases. This reliable alternative facilitates research in optical diagnostics or biosensor development by simplifying preliminary sensor testing.
1. Introduction
Contemporary science addresses increasing challenges by developing safe, effective, and ethical research methods. One significant development is phantoms—artificially created materials that simulate the properties of biological counterparts [1,2]. In this context, the development of a urine phantom that accurately replicates the ultraviolet–visible (UV–Vis) spectrum of human urine provides new opportunities in medical diagnostics and the validation of analytical technologies. Urine diagnostics plays a crucial role in medicine, as it is a non-invasive method that enables the detection of a wide range of diseases, including urinary tract infections, diabetes, kidney disorders, and even early cancer detection [3,4,5]. Optical methods, such as UV–Vis spectroscopy, enable the accurate identification of biomarkers present in urine [6,7,8].
The use of phantoms offers significant practical and technical advantages. Utilizing biological materials in research requires adherence to safety protocols, specific storage conditions, and obtaining bioethical approvals, which require substantial costs and limit the availability of such samples. Phantoms address these issues by providing an alternative that reduces the risks associated with exposure to potentially hazardous samples and enables research under standard laboratory conditions without the need for highly specialized personnel [9]. Furthermore, phantoms are durable, easy to store, and simple to produce, which further enhances their practical utility.
This study aims to develop a simple, cost-effective, and safe urine phantom whose optical properties in the UV–Vis range closely replicate those of healthy human urine [10,11]. This study involved the creation of a urine phantom with optical properties resembling real urine in the UV–Vis spectrum, as well as an analytical component, consisting of a comparison of its spectrum with biological samples. This comparison results provide the basis for further research into the potential applications of this phantom in analytical technology. It is worth emphasizing that, to date, no urine phantom has been developed that unequivocally reproduces the UV–Vis spectrum of biological samples. Therefore, the presented phantom represents an innovative solution that can be applied in research on diagnostic sensors and the validation of optical analytical methods.
The proposed phantom was prepared using only four ingredients, which are widely available, inexpensive, and safe, making it easy to reproduce in any laboratory. The composition of the phantom was optimized to achieve a UV–Vis spectrum that closely replicates the characteristic spectrum of human urine. Due to this high level of conformity, the phantom can be effectively used for the validation of spectroscopic methods and testing sensors under laboratory conditions before their application in clinical diagnostics. Additionally, the phantom enables preliminary studies to be conducted quickly and cost-effectively, contributing to the acceleration of work on new diagnostic technologies.
The uniqueness of the developed phantom lies in its simplicity, minimal number of components, low production cost [12,13], and high compatibility with the UV–Vis spectrum of biological samples. This innovative solution enables preliminary experiments for further research into advanced models that simulate additional biological properties of urine, such as pH variability, ionic composition, or the presence of disease biomarkers. The developed phantom represents a significant step in creating easily accessible diagnostic tools, supporting the advancement of optical and sensor technologies in medical and analytical research [13,14,15].
2. Materials and Methods
2.1. Materials
To create artificial urine for sensor validation, the key components of the spectra must be replicated. The formula included water as the base, which was also the base of artificial urine. Another important component was mineral salts, which were obtained by adding a phosphate-buffered saline (PBS) solution (Sigma-Aldrich Co, Saint Louis, MO, USA). Urea and uric acid are other crucial elements of urine, and the absorbance is dominated by acid. In the artificial urine, we added citric acid (Sigma-Aldrich Co, Saint Louis, USA) and a mix of acetylsalicylic acid and ascorbic acid (Bayer AG, Leverkusen, Germany). These acids are readily available (in products like aspirin), and safer compared to other acids, and their spectral properties closely resemble those of uric acid. To enhance the color to resemble natural urine and improve the absorption spectrum, a black tea infusion (Mokate, Ustron, Poland) was added at the final stage. The black tea provided a color similar to urine. The caffeine present in the infusion improved the absorption spectrum in a range of 250–300 nm. The caffeine content depends on the time of brewing.
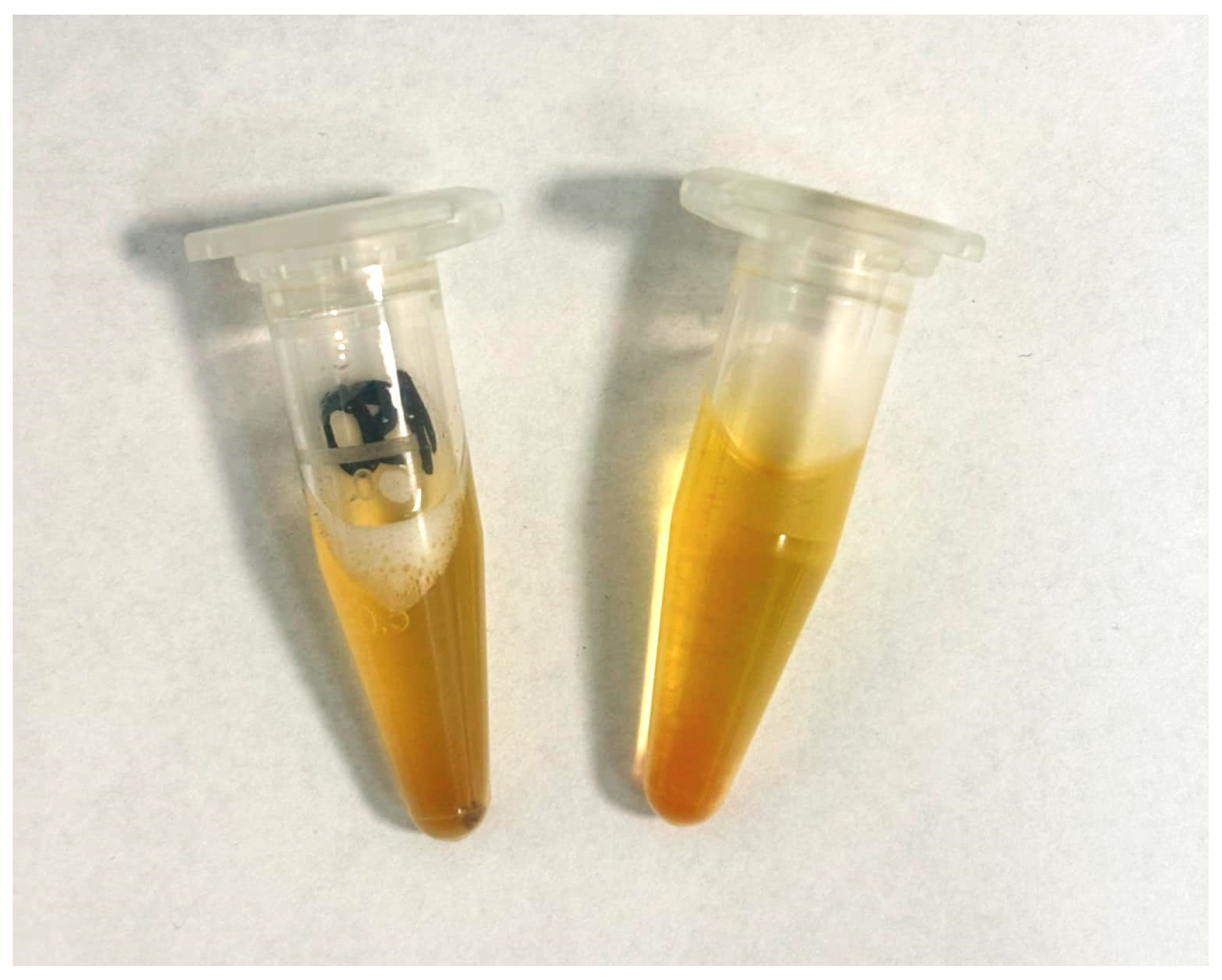
The components were selected for their easy availability and safety. The phantom was fabricated using only four components, highlighting its simplicity and accessibility. Four components were used: PBS solution (Sigma-Aldrich, USA), an aspirin tablet (Bayer, Germany), citric acid (Sigma-Aldrich, USA), and a tea infusion (Mokate, Poland) [16]. This allowed a phantom to be reproduced quickly and cheaply in any laboratory, without the need for complex procedures. The resulting product of combining the above components is shown in Figure 1. The order in which the ingredients were added was not important. The proportions and concentrations of the individual components were crucial. The developed phantom exhibited a color and appearance that closely mimicked that of natural urine, making it suitable for visual and spectroscopic studies requiring realistic substitutes.
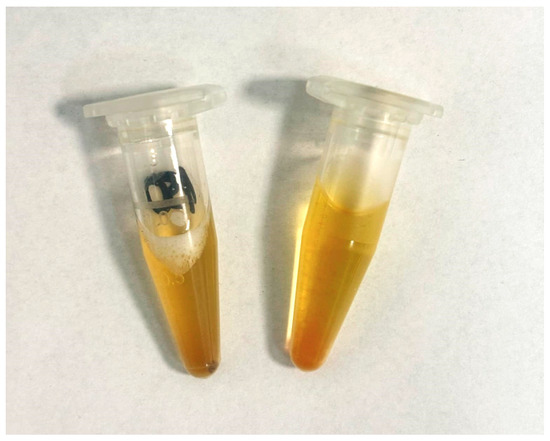
Figure 1.
Developed urine phantom in this study.
2.2. Method
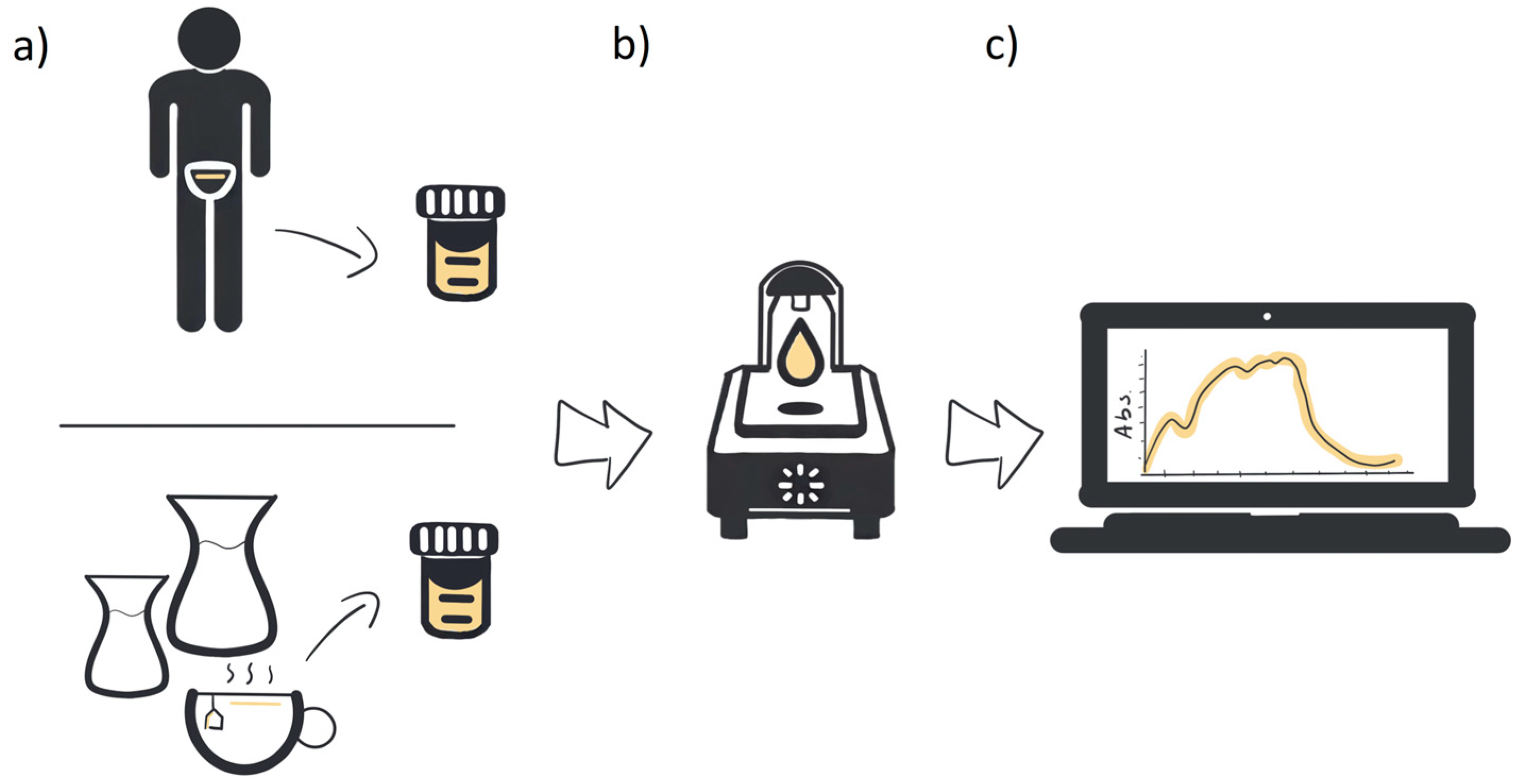
For absorbance measurement, we used a Nanodrop One C (Thermo Fisher Scientific Inc., Waltham, MA, USA) spectrometer. The wavelength range was 190–850 nm with a photometric accuracy of 3% at 302 nm. The bandwidth was ≤1.8 nm. The measuring device was used to detect materials at low concentrations. A sample volume of 2 µL was used for each measurement. All measurements were conducted at room temperature. For the measurements, urine from a patient and artificially produced urine were used. After the measurements, the obtained spectra were compared by checking the coincidence of the peaks (Figure 2).
Figure 2.
Schematic diagram of the method: (a) patient’s sample or phantom fabrication, (b) spectroscopic measurements, and (c) spectra comparison.
3. Results and Discussion
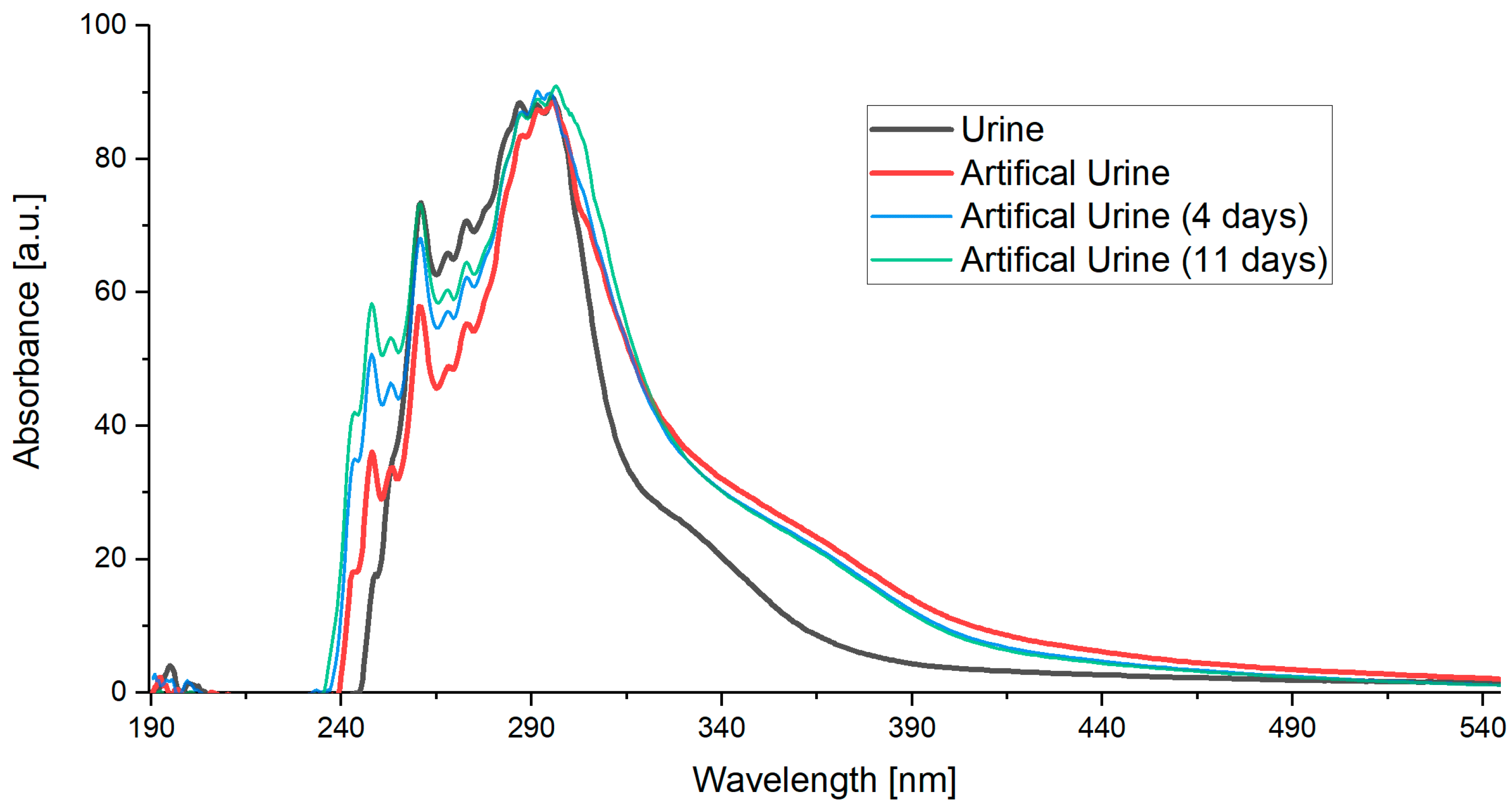
The measured results (Figure 3) showed that the artificial urine closely matched the spectra of real human urine across the UV and visible range. The main peaks aligned with differences only in signal intensity.
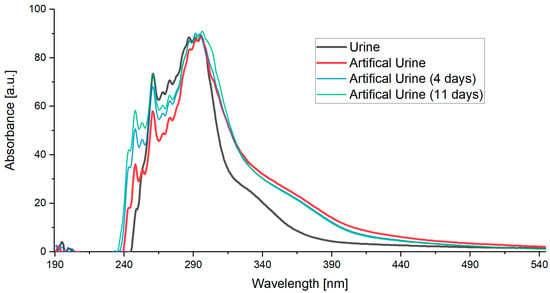
Figure 3.
Obtained spectra of urine and artificial urine.
The spectrum from each patient varied slightly due to different concentrations, depending on the patient’s condition, diet, and medication intake. By adjusting the concentration of individual components in the artificial urine, it was possible to make slight adaptations to match the conditions of specific patients. Also, changes occurring in the phantom over time were tested. Spectral variations were evident in the signal intensity. Notably, absorption values increased in the range of 240–280 nm. In this way, the phantom’s spectra were adjusted to the urine spectrum. However, this is not essential for sensor validation, as the relevant range of interest changes only marginally.
4. Conclusions
Artificial urine was formulated that accurately reflected the UV–Vis spectrum. We achieved the desired spectral range using only a few components, which is a significant accomplishment considering the complexity and number of constituents in natural urine. We did not replicate the chemical composition of natural urine, as the quantity and diversity of its components, as well as their availability, make it challenging to reproduce. The formulated phantom mirrors the optical characteristics of natural urine and provides a consistent and reproducible medium for experimental research. It can be adapted to create more advanced phantoms tailored to the specific optical properties of individual patients or particular diseases.
Author Contributions
Conceptualization, P.S. and M.B.; methodology, P.S. and M.B.; investigation, P.S. and M.B.; writing—original draft preparation, P.S. and M.B.; writing—review and editing, P.S. and M.B.; visualization, P.S. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by DS programs of the Faculty of Electronics, Telecommunications and Informatics of Gdańsk Tech, by the 14/1/2024/IDUB/III.4.1/Tc and 7/1/2024/IDUB/III.4c/Tc grants under the TECHNETIUM Talent Management Grants program at Gdańsk Tech and COST (European Cooperation in Science and Technology) [CA21159].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request to interested researchers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suardi, N.; Ahmad, M.; Shukri, A.; Mohammad, H.; Oglat, A.; Alarab, A.; Makhamrah, O. Chemical characteristics, motivation and strategies in choice of materials used as liver phantom: A literature review. J. Med. Ultrasound 2020, 28, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Karpienko, K.; Gnyba, M.; Milewska, D.; Wróbel, M.S.; Jȩdrzejewska-Szczerska, M. Blood Equivalent Phantom vs Whole Human Blood, a Comparative Study. J. Innov. Opt. Health Sci. 2016, 9. [Google Scholar] [CrossRef]
- Urinalysis: A Wealth of Information in Just a Few Drops. Available online: https://www.siemens-healthineers.com/perspectives/urinalysis-a-wealth-of-information (accessed on 27 December 2024).
- Władziński, A.; Kosowska, M.; Wityk, P.; Łuczkiewicz, A.; Gnyba, M.; Szczerska, M. Biomarker Detection in the Wastewater Phantom. J. Biophotonics 2025, 13, e202500003. [Google Scholar] [CrossRef] [PubMed]
- Babińska, M.; Władziński, A.; Talaśka, T.; Szczerska, M. Machine Learning Enhanced Optical Fiber Sensor for Detection of Glucose Low Concentration in Samples Mimicking Tissue. Photon-Lett. Pol. 2025, 17, 20–22. [Google Scholar] [CrossRef]
- Alijaj, N.; Pavlovic, B.; Martel, P.; Rakauskas, A.; Cesson, V.; Saba, K.; Hermanns, T.; Oechslin, P.; Veit, M.; Provenzano, M.; et al. Identification of Urine Biomarkers to Improve Eligibility for Prostate Biopsy and Detect High-Grade Prostate Cancer. Cancers 2022, 14, 1135. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, A.; Ngajikin, N.H.; Rashid, N.C.A.; Ali, S.H.A.; Yaacob, M.; Isaak, S.; Cholan, N.A. Uric Acid Detection in Visible Spectrum. TELKOMNIKA Telecommun. Comput. Electron. Control. 2020, 18, 2035–2041. [Google Scholar] [CrossRef]
- Sokolowski, P.; Wityk, P.; Cierpiak, K.; Babińska, M.; Graczyk, W.; Krawczyk, B.; Markuszewski, M.; Szczerska, M. Optical method supported by machine learning for urinary tract infections discrimination and bladder cancer detection. In Proceedings of the Optical Sensing and Detection VIII, Strasbourg, France, 20 June 2024; p. 85. [Google Scholar]
- Gorpas, D.; Wabnitz, H.; Pfefer, T.J. Special Section Guest Editorial: Tissue Phantoms to Advance Biomedical Optical Systems. J. Biomed. Opt. 2022, 27, 074701. [Google Scholar] [CrossRef] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Sarigul, N.; Korkmaz, F.; Kurultak, I. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef] [PubMed]
- Sieryi, O.; Popov, A.P.; Kalchenko, V.; Bykov, A.V.; Meglinski, I. Tissue-mimicking phantoms for biomedical applications. In Proceedings of the Tissue Optics and Photonics, Online, 12 May 2020; p. 1136312. [Google Scholar]
- Esmonde-White, F.W.L.; Esmonde-White, K.A.; Kole, M.R.; Goldstein, S.A.; Roessler, B.J.; Morris, M.D. Biomedical tissue phantoms with controlled geometric and optical properties for Raman spectroscopy and tomography. Analyst 2011, 136, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Cuper, N.J.; Klaessens, J.H.G.; Jaspers, J.E.N.; de Roode, R.; Noordmans, H.J.; de Graaff, J.C.; Verdaasdonk, R.M. The use of near-infrared light for safe and effective visualization of subsurface blood vessels to facilitate blood withdrawal in children. Med. Eng. Phys. 2013, 35, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.; Keevil, C.W. A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 1997, 3, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Babińska, M.; Władziński, A. Application of UV-VIS Spectroscopy and Machine Learning Methods in Glucosuria Diagnostics: A Phantom Study. Photonics Lett. Pol. 2025, 17, 16–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).