Utilizing ZSM-5 Zeolite, Synthesized from Kaolin Clay, as a Catalyst Presents an Efficient Approach for Reducing Emissions in Compression Ignition (CI) Engines †
Abstract
1. Introduction
2. Methodology
2.1. Synthesis of Kaolin Clay
2.2. Zeolite
2.3. Properties of Zeolite
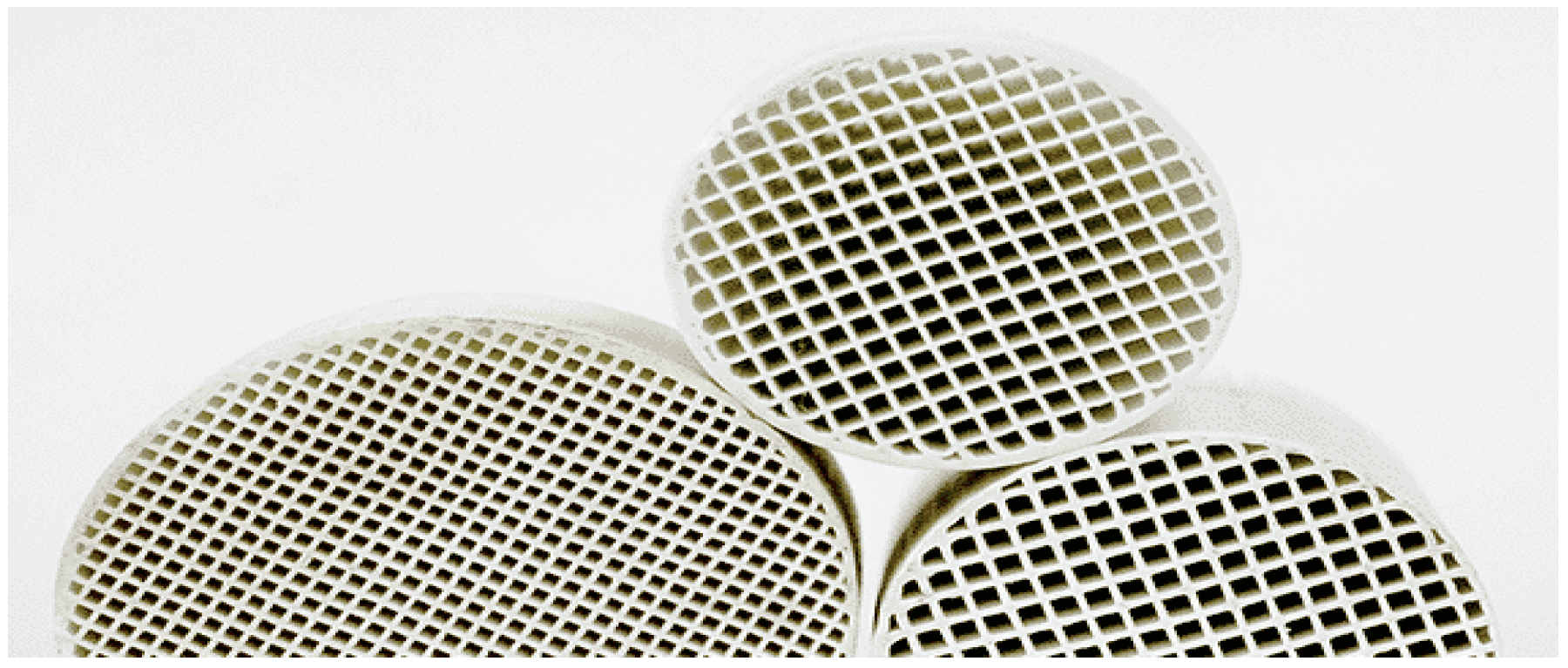
2.4. Cordierite Monolith
2.5. Preparation of Catalyst
2.5.1. Na+ Ion Exchange Method
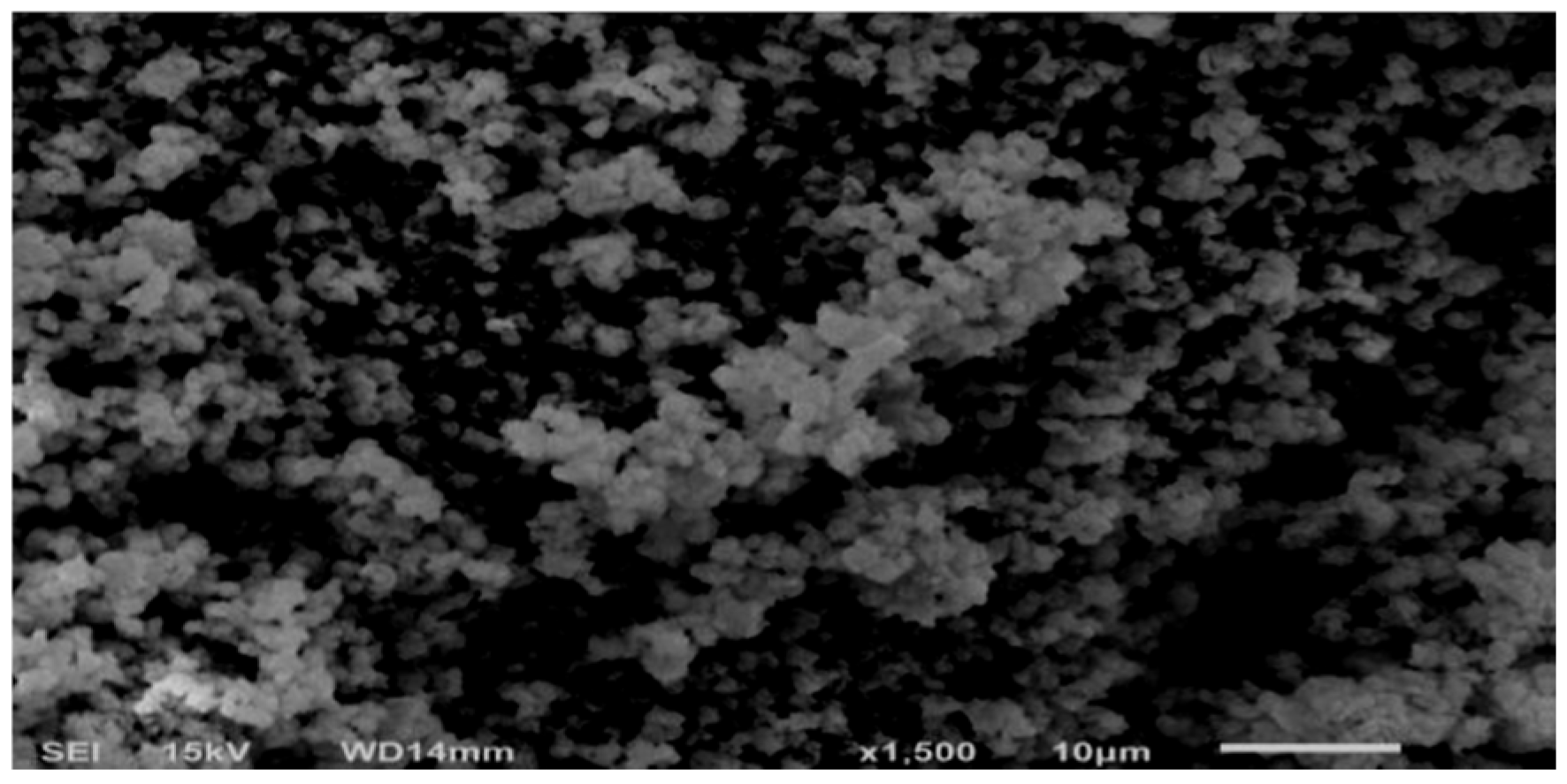
2.5.2. Catalyst Characterization—SEM
2.5.3. Catalyst Characterization—XRD
3. Experimentation
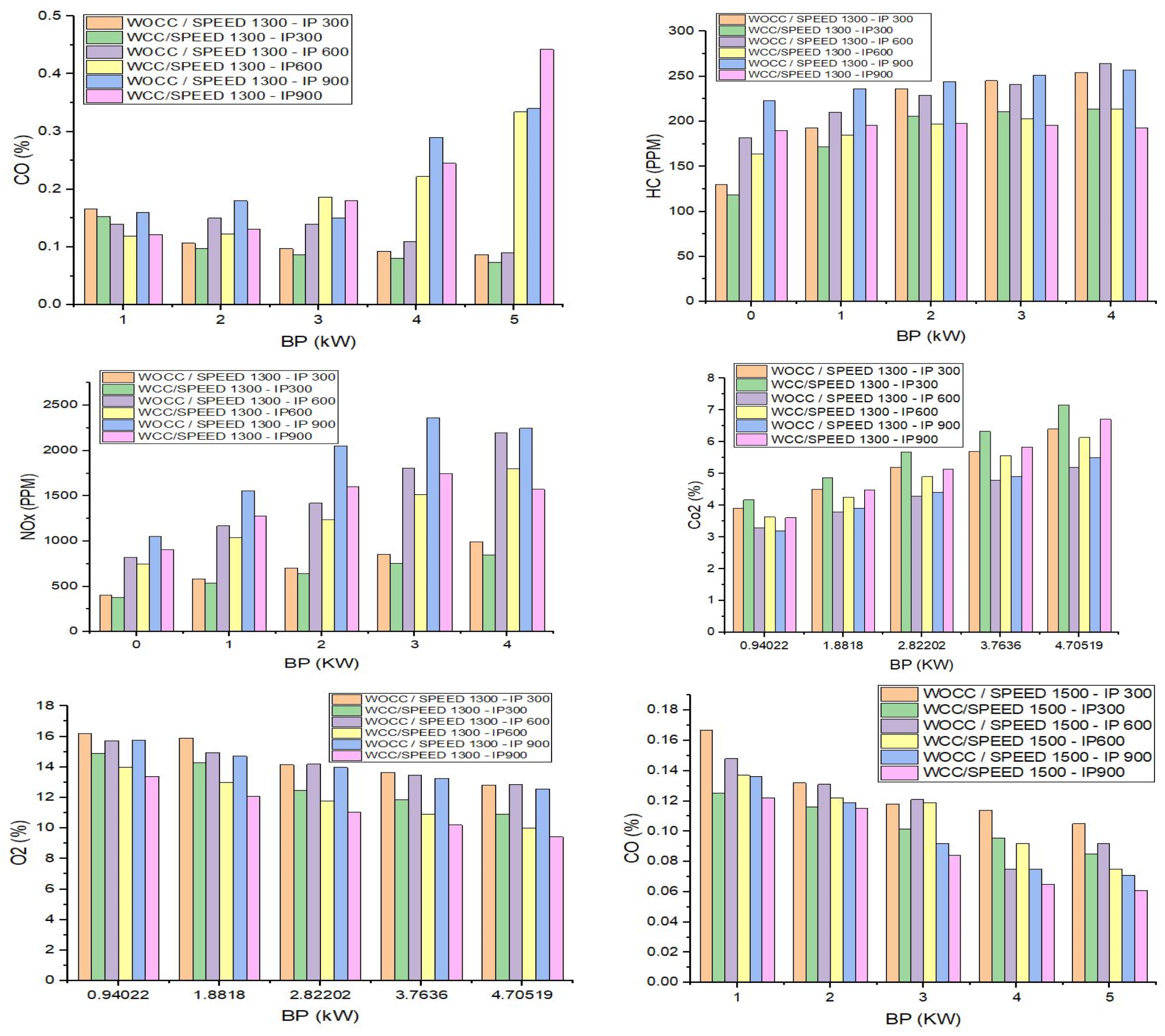
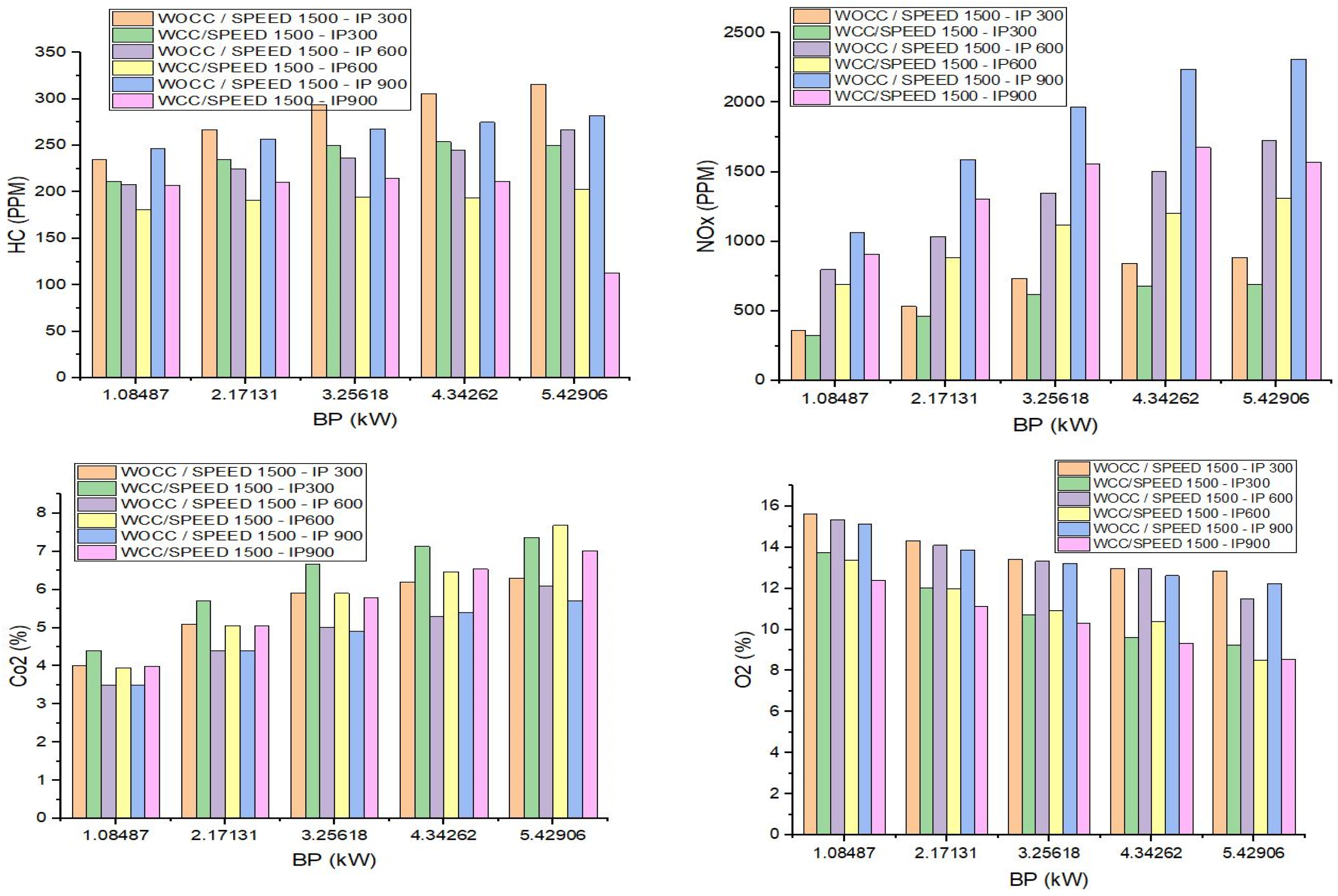
4. Emission Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, T.V.; Rao, G.P.; Reddy, K.H.C. Experimental Investigation of Pongamia, Julifra and Neem Methyl Esters as Biodiesel on C.I. Engine. Jordan J. Mech. Ind. Eng. 2008, 2, 117–122. [Google Scholar]
- Reddy, V.S.; Ranjan, K.R.; Sharma, V.K.; Tyagi, S.K. Experimental investigation of a diesel engine fuelled with Julifra curcas L. seed oil. Int. J. Sustain. Energy 2011, 30, S4–S10. [Google Scholar] [CrossRef]
- Damanik, N.; Ong, H.C.; Tong, C.W.; Mahlia, T.M.; Silitonga, A.S. A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ. Sci. Pollut. Res. 2018, 25, 15307–15325. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Geng, L.M.; Bian, Y.Z.H. Experimental studies on the combustion characteristics and performance of a direct injection engine fueled with biodiesel/diesel blends. Energy Convers. Manag. 2010, 51, 2985–2992. [Google Scholar] [CrossRef]
- Pushparaj, T.; Ramabalan, S.; Selvan, V.A. Performance and emission characteristics of CI engine, fuelled with diesel and oxygenated fuel blends. Int. J. Glob. Warm. 2015, 7, 173. [Google Scholar] [CrossRef]
- Thamizhvel, R.; Sethuraman, N.; Sakthivel, M.; Prabhakaran, R. Experimental investigation of diesel engine by using paper cup waste as the producer gas with help of down-draft gasifier. IOP Conf. Ser. Mater. Sci. Eng. 2020, 988, 012015. [Google Scholar] [CrossRef]
- Rajakrishnamoorthy, P.; Saravanan, C.G.; Natarajan, R.; Karthikeyan, D.; Sasikala, J.; Josephin, J.S.F.; Vikneswaran, M.; Sonthalia, A.; Varuvel, E.G. Exhaust emission control of SI engines using ZSM-5 zeolite supported bimetals as a catalyst synthesized from coal fly ash. Fuel 2023, 340, 127380. [Google Scholar] [CrossRef]
- Sethuraman, N.; Varman, K.S.; Venkatakrishnan, R.; Thamizhvel, R. An experimental investigation of crude glycerol into useful products by using IC engine in dual fuel mode. Mater. Today Proc. 2021, 44, 3914–3918. [Google Scholar] [CrossRef]
- Thamizhvel, R.; Suryavarman, K.; Velmurugan, V.; Sethuraman, N. Comparative study of gasification and pyrolysis derived from coconut shell on the performance and emission of CI engine. Mater. Today Proc. 2021, 47, 978–983. [Google Scholar] [CrossRef]
- Karthikeyan, D.; Saravanan, C.G. Experimental analysis of flyash based, ion exchanged zeolite as catalyst for S.I engine emission control. J. KONES Powertrain Transp. 2013, 20, 229–235. [Google Scholar]
- Sethuraman, N.; Karthikeyan, D. Investigation of emission reduction in CI engine by using CuCl2, Cucl2, MgCl2 and FeCl2 doped ZSM5 as catalyst. Mater. Today Proc. 2022, 62, 858–862. [Google Scholar] [CrossRef]
- Ramalingam, S.; Thamizhvel, R.; Sudagar, S.; Silambarasan, R. Production of third generation bio-fuel through thermal cracking process by utilizing COVID-19 plastic wastes. Mater. Today Proc. 2023, 72, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, N.; Karthikeyan, D.; Vinodraj, S.; Thamizhvel, R.; Kumarasamy, G. Performance and emission characteristics on CI engine by using waste cooking oil and waste transformer oil as substitute fuel with coconut shell as a catalyst. Mater. Today Proc. 2023, 72, 2469–2475. [Google Scholar] [CrossRef]
- Senthil, R.; Thamizhvel, R.; Sudagar, S. A comparative analysis of pyrolytic oil from medical waste and its suitability as fuel in a Compression Ignition engine. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 2040–2058. [Google Scholar] [CrossRef]
- Thamizhvel, R.; Manikandan, A.; Praveen Kumar, S.; Sivasankaran, S. Potential Use of Cathode Ray Tube as an Abrasive Particle in Abrasive Jet Machine. In Proceedings of the 2023 International Conference on Energy, Materials and Communication Engineering (ICEMCE), Madurai, India, 14–15 December 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Thamizhvel, R.; Irine, G.S.M.; Vaithianathan, N.; Ganesh, M. Evaluating the performance and emission characteristics of jackfruit seed as bio-oil in CI engine. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Karthikeyan, D. Research on Metal Doped Zeolite as Catalyst to Reduce NOX Emission from Lean Burn Gasoline Engines. Int. J. Eng. Adv. Technol. 2019, 8, 201–206. [Google Scholar] [CrossRef]
- Karthikeyan, D.; Saravanan, C.; Jeyakumar, T. Catalytic Reduction of S.I. Engine Emissions Using Zeolite as Catalyst Synthesized from Coal Fly Ash. Int. J. Eng. Technol. 2016, 6, 62–68. [Google Scholar]
- Rajakrishnamoorthy, P.; Saravanan, C.G.; Karthikeyan, D. Emission reduction technique applied in ci engine exhaust using zsm5 zeolite as catalyst. Pollut. Res. Pap. 2019, 38, 1041–1047. [Google Scholar]
- Senthil, R.; Thamizhvel, R.; Sudagar, S.; Bahuruteen Ali Ahamadu, H. Production of high-grade fuels from biomass and waste plastics and its influence in a CI engine. Polym. Bull. 2025, 82, 6095–6114. [Google Scholar] [CrossRef]
- Ramalingam, S.; Rajkumar, T.; Subramanian, S.; Palani, S. Investigation of combustion, emission, and performance parameters of a natural antioxidant additives using hydrogen and biodiesel as dual fuel in CI engine operation. Int. J. Hydrog. Energy 2024, 110, 44–54. [Google Scholar] [CrossRef]
- Rajakrishnamoorthy, P.; Elavarasan, G.; Karthikeyan, D.; Saravanan, C. Emission Reduction in SI Engines by using metal doped Cu- ZSM5 and Ce.Cu- ZSM5 zeolite as Catalysts. Int. J. Innov. Technol. Explor. Eng. (IJITEE) 2019, 8, 1423–1427. [Google Scholar]



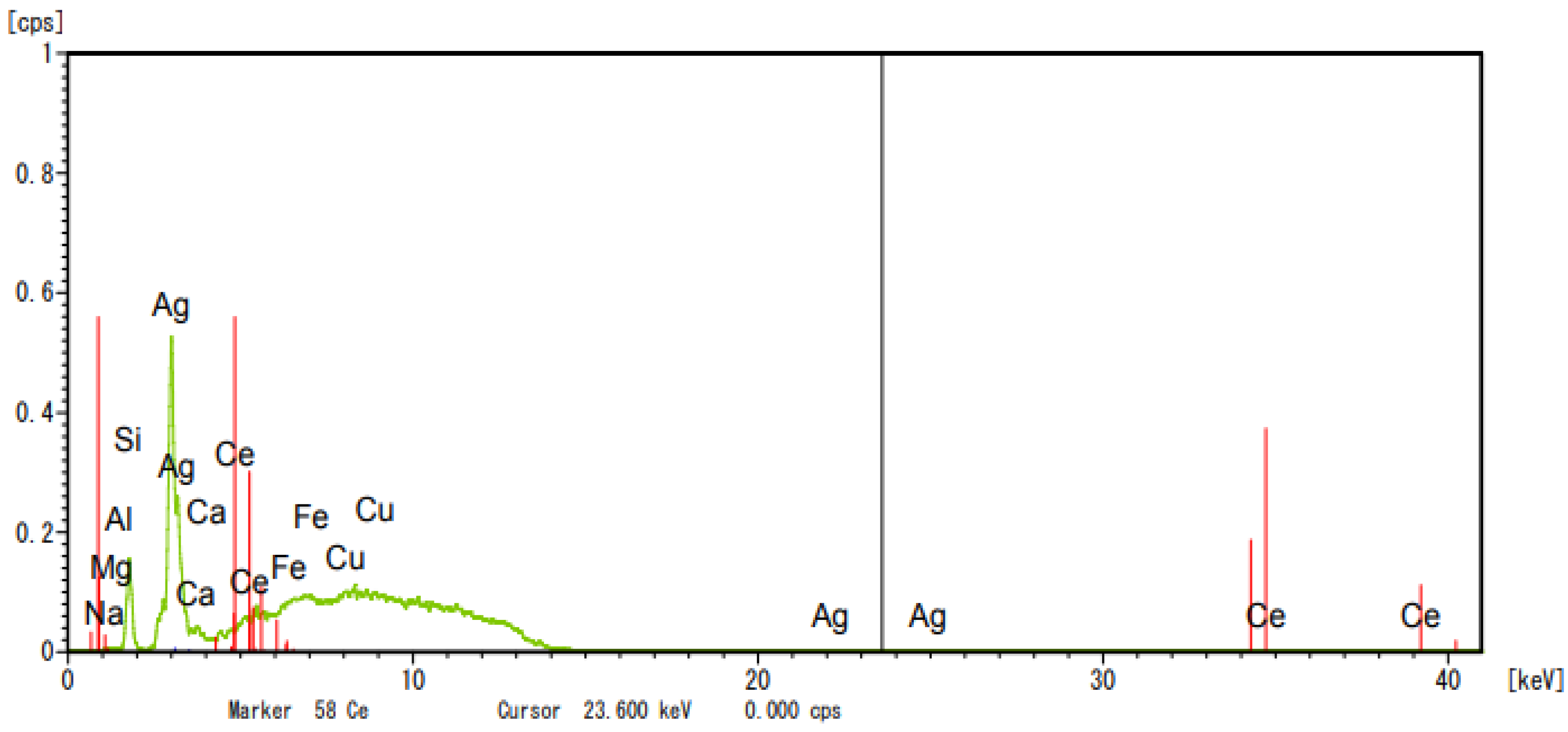
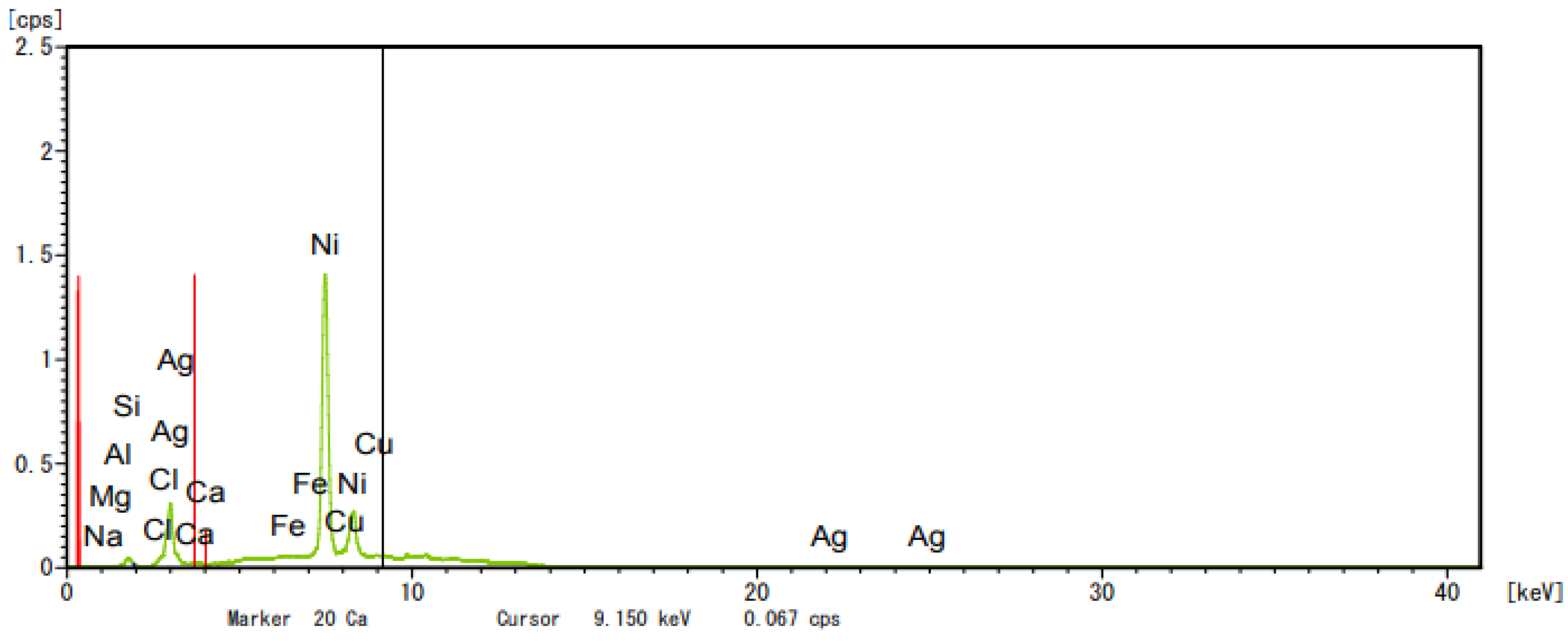

| Composition | ZSM5 | Cucl2-ZSM5 | AgNO3-ZSM5 |
|---|---|---|---|
| Sio2 | 70.71 | 70.07 | 67.633 |
| Al2O3 | 6.32 | 7.26 | 8.722 |
| Fe2O3 | 0.93 | 0.00 | 0.75 |
| caO | 0.91 | 0.00 | 0.25 |
| MgO | 1.11 | 0.00 | 0.62 |
| SO2 | 1.17 | 0.00 | 0.01 |
| Na2O | 14.35 | 18.33 | 19.28 |
| K2O | 0.87 | 0.00 | 1.92 |
| CuO | 1.12 | 10.24 | 0.00 |
| P2O5 | 1.27 | 0.00 | 0.612 |
| TiO2 | 0.29 | 0.00 | 0.10 |
| AgO | 0.00 | 0.00 | 10.23 |
| Particulars | Details |
|---|---|
| Make/Model | Mahindra Maximo |
| Bore | 83 mm |
| Stroke | 84 mm |
| Type | Common Rail Direct Injection |
| Cooling Type | Water |
| Displacement (Swept Volume) | 909 cc |
| Fuel | Diesel |
| Speed | 2000 rpm |
| Torque | 50 Nm |
| Maximum Load in Dynamometer Load cell | 18 kgs |
| Starting Type | Electric Start |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, S.; Duraisamy, K.; Bharanitharan, A. Utilizing ZSM-5 Zeolite, Synthesized from Kaolin Clay, as a Catalyst Presents an Efficient Approach for Reducing Emissions in Compression Ignition (CI) Engines. Eng. Proc. 2025, 93, 16. https://doi.org/10.3390/engproc2025093016
Narayanan S, Duraisamy K, Bharanitharan A. Utilizing ZSM-5 Zeolite, Synthesized from Kaolin Clay, as a Catalyst Presents an Efficient Approach for Reducing Emissions in Compression Ignition (CI) Engines. Engineering Proceedings. 2025; 93(1):16. https://doi.org/10.3390/engproc2025093016
Chicago/Turabian StyleNarayanan, Sethuraman, Karthikeyan Duraisamy, and Aasthiya Bharanitharan. 2025. "Utilizing ZSM-5 Zeolite, Synthesized from Kaolin Clay, as a Catalyst Presents an Efficient Approach for Reducing Emissions in Compression Ignition (CI) Engines" Engineering Proceedings 93, no. 1: 16. https://doi.org/10.3390/engproc2025093016
APA StyleNarayanan, S., Duraisamy, K., & Bharanitharan, A. (2025). Utilizing ZSM-5 Zeolite, Synthesized from Kaolin Clay, as a Catalyst Presents an Efficient Approach for Reducing Emissions in Compression Ignition (CI) Engines. Engineering Proceedings, 93(1), 16. https://doi.org/10.3390/engproc2025093016






