Rheological Properties of Functionalized Smart Resins for Transport Applications †
Abstract
1. Introduction
2. Materials and Methods
3. Results
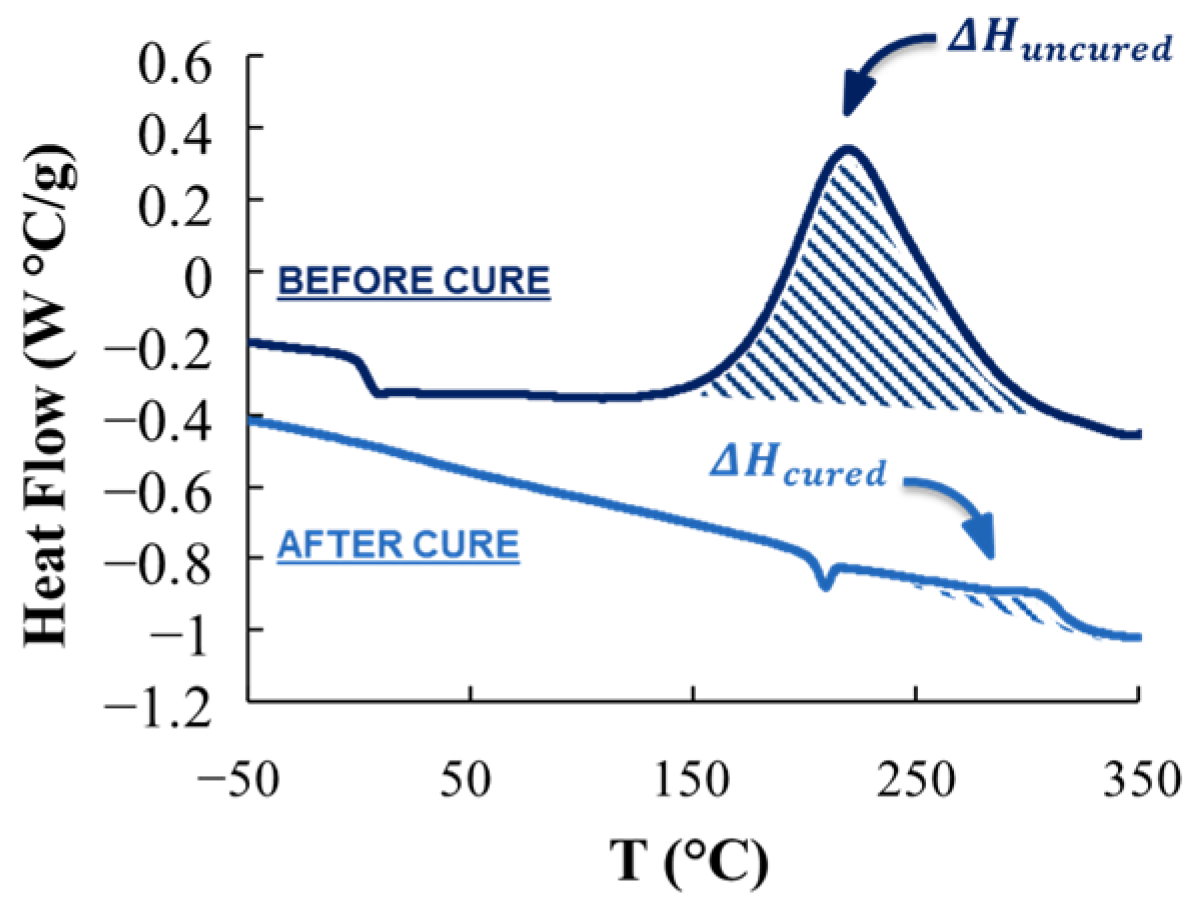
3.1. Thermal Characterization
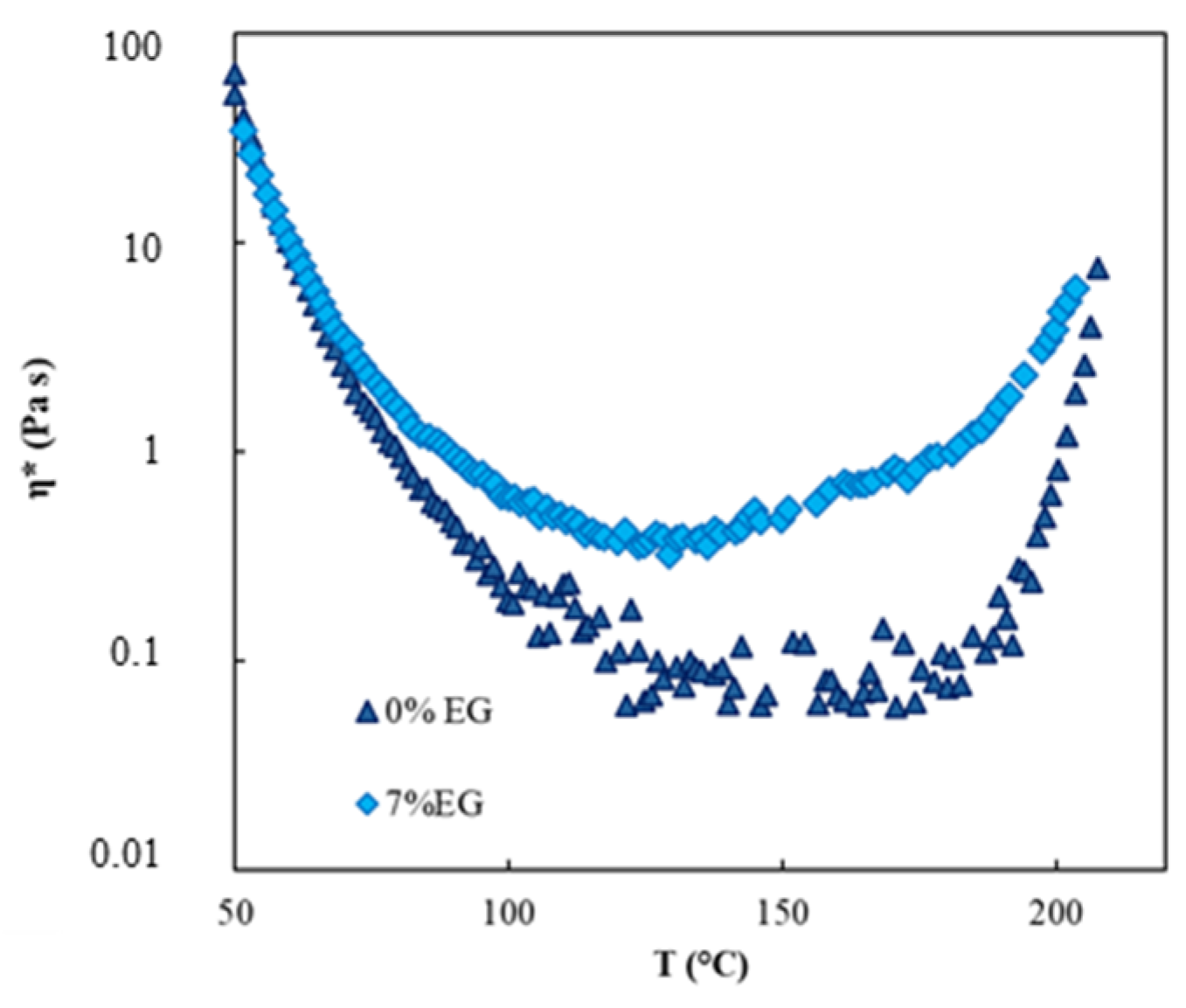
3.2. Rheological Characterization
3.3. Post-Cure Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, M.P.; Gul, W.; Cimini Junior, C.A.; Ha, S.K. A Review on Industrial Perspectives and Challenges on Material, Manufacturing, Design and Development of Compressed Hydrogen Storage Tanks for the Transportation Sector. Energies 2022, 15, 5152. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Air, A.; Shamsuddoha, M.; Gangadhara Prusty, B. A Review of Type V Composite Pressure Vessels and Automated Fibre Placement Based Manufacturing. Compos. Part B Eng. 2023, 253, 110573. [Google Scholar] [CrossRef]
- Condé-Wolter, J.; Ruf, M.G.; Liebsch, A.; Lebelt, T.; Koch, I.; Drechsler, K.; Gude, M. Hydrogen Permeability of Thermoplastic Composites and Liner Systems for Future Mobility Applications. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107446. [Google Scholar] [CrossRef]
- Guadagno, L.; Longo, R.; Aliberti, F.; Lamberti, P.; Tucci, V.; Pantani, R.; Spinelli, G.; Catauro, M.; Vertuccio, L. Role of MWCNTs Loading in Designing Self-Sensing and Self-Heating Structural Elements. Nanomaterials 2023, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.C.; Biga, R.; Kolisnichenko, A.; Marocco, P.; Monteverde, A.H.A.; Santarelli, M. Potential and Technical Challenges of On-Board Hydrogen Storage Technologies Coupled with Fuel Cell Systems for Aircraft Electrification. J. Power Sources 2023, 555, 232397. [Google Scholar] [CrossRef]
- Vertuccio, L.; Calabrese, E.; Raimondo, M.; Catauro, M.; Sorrentino, A.; Naddeo, C.; Longo, R.; Guadagno, L. Effect of Temperature on the Functionalization Process of Structural Self-Healing Epoxy Resin. Aerospace 2023, 10, 476. [Google Scholar] [CrossRef]
- Guadagno, L.; Sorrentino, A.; Longo, R.; Raimondo, M. Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites. Polymers 2023, 15, 2297. [Google Scholar] [CrossRef] [PubMed]
- Nachtane, M.; Tarfaoui, M.; Abichou, M.a.; Vetcher, A.; Rouway, M.; Aâmir, A.; Mouadili, H.; Laaouidi, H.; Naanani, H. An Overview of the Recent Advances in Composite Materials and Artificial Intelligence for Hydrogen Storage Vessels Design. J. Compos. Sci. 2023, 7, 119. [Google Scholar] [CrossRef]
- Di, C.; Yu, J.; Wang, B.; Lau, A.K.T.; Zhu, B.; Qiao, K. Study of Hybrid Nanoparticles Modified Epoxy Resin Used in Filament Winding Composite. Materials 2019, 12, 3853. [Google Scholar] [CrossRef] [PubMed]
- Keyte, J.; Pancholi, K.; Njuguna, J. Recent Developments in Graphene Oxide/Epoxy Carbon Fiber-Reinforced Composites. Front. Mater. 2019, 6, 224. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Meng, Y. Curing of DGEBA Epoxy Using a Phenol-Terminated Hyperbranched Curing Agent: Cure Kinetics, Gelation, and the TTT Cure Diagram. Thermochim. Acta 2012, 549, 69–80. [Google Scholar] [CrossRef]




| Filler Amount (%) | αmax (%) |
|---|---|
| 0 | 100.0 |
| 1 | 98.4 |
| 3 | 97.9 |
| 7 | 95.8 |
| 9 | 94.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piano, G.D.; Longo, R.; Guadagno, L.; Pantani, R. Rheological Properties of Functionalized Smart Resins for Transport Applications. Eng. Proc. 2025, 90, 6. https://doi.org/10.3390/engproc2025090006
Piano GD, Longo R, Guadagno L, Pantani R. Rheological Properties of Functionalized Smart Resins for Transport Applications. Engineering Proceedings. 2025; 90(1):6. https://doi.org/10.3390/engproc2025090006
Chicago/Turabian StylePiano, Giorgia De, Raffaele Longo, Liberata Guadagno, and Roberto Pantani. 2025. "Rheological Properties of Functionalized Smart Resins for Transport Applications" Engineering Proceedings 90, no. 1: 6. https://doi.org/10.3390/engproc2025090006
APA StylePiano, G. D., Longo, R., Guadagno, L., & Pantani, R. (2025). Rheological Properties of Functionalized Smart Resins for Transport Applications. Engineering Proceedings, 90(1), 6. https://doi.org/10.3390/engproc2025090006









