Conceptual Design of a Metal Hydride System for the Recovery of Gaseous Hydrogen Boil-Off Losses from Liquid Hydrogen Tanks †
Abstract
1. Introduction
2. LH2 Handling and Boil-Off
2.1. Review of LH2 Handling at Airports
2.2. Boil-Off Recovery by Metal Hydrides
- No trans-sectoral use is considered, like BOG capture from an aircraft’s LH2 tank and a subsequent consumption in a ground-based vehicle;
- Limited to narrow operation windows and therefore not suited for varying pressure levels of different boil-off sources and several potential consumers;
- No mobile use is intended to enable distribution or intermediate hydrogen storage according to the needs of the processes at the airport.
3. Methodology of the Conceptual Design
4. Results of the Conceptual Design
4.1. Requirements
4.2. Functional Structure Tree and Corresponding Working Principles
4.2.1. Interface Design Proposals
4.2.2. Design Proposals for the TMS of the MH Cartridge
4.2.3. Proposals for MH Cartridge Logistics
4.3. Combination of Working Principles to a Design Concept
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive Investigation on Hydrogen and Fuel Cell Technology in the Aviation and Aerospace Sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Stephan, T.; Breiteneder, T.; Winklhofer, J. Comparison of Liquified Gas Energy Carriers and Conventional Fossil Fuels with a Focus on Storage Requirements for the Use in Mobile Applications. In Proceedings of the Vienna Motor Symposium, Vienna, Austria, 26–28 April 2023. [Google Scholar]
- Huete, J.; Pilidis, P. Parametric Study on Tank Integration for Hydrogen Civil Aviation Propulsion. Int. J. Hydrogen Energy 2021, 46, 37049–37062. [Google Scholar] [CrossRef]
- Warwick, N.; Griffiths, P.; Keeble, J.; Archibald, A.; Pyle, J.; Shine, K. Atmospheric Implications of Increased Hydrogen Use; Department for Energy Security and Net Zero and Department for Business, Energy & Industrial Strategy: London, UK, 2022. Available online: https://www.gov.uk/government/publications/atmospheric-implications-of-increased-hydrogen-use (accessed on 27 April 2022).
- Mangold, J.; Silberhorn, D.; Moebs, N.; Dzikus, N.; Hoelzen, J.; Zill, T.; Strohmayer, A. Refueling of LH2 Aircraft—Assessment of Turnaround Procedures and Aircraft Design Implication. Energies 2022, 15, 2475. [Google Scholar] [CrossRef]
- Petitpas, G. Boil-Off Losses Along LH2 Pathway; LLNL-TR-750685; LLNL: Livermore, CA, USA, 2018.
- Brewer, G.D. Hydrogen Aircraft Technology, 1st ed.; CRC Press LLC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Turner, J.; Contaut, S.; Tarantino, A.; Masson, A. FlyZero: Cryogenic-Hydrogen-Fuel-System-and-Storage-Roadmap-Report; Aerospace Technology Institute: Cranfield, UK, 2022; Available online: https://www.ati.org.uk/flyzero-reports/ (accessed on 25 July 2022).
- Franke, F.; Kazula, S.; Enghardt, L. Elaboration and Outlook for Metal Hydride Applications in Future Hydrogen-powered Aviation. Aeronaut. J. 2024, 128, 1501–1531. [Google Scholar] [CrossRef]
- Pohl, G. Konzeptentwicklung zum Auffangen und Verwerten von Gasförmigen Wasserstoffverlusten aus dem Boil-Off von Flüssigwasserstofftanks Durch Metallhydrid. Bachelor’s Thesis, BTU Cottbus-Senftenberg, Cottbus, Germany, 2024. [Google Scholar]
- Rosso, M.J.; Golben, P.M. Capture of Liquid Hydrogen Boiloff with Metal Hydride Absorbers; NASA CR 83098; John F. Kennedy Space Center: Merritt Island, FL, USA, 1984.
- Kölbig, M.; Bürger, I.; Linder, M. Characterization of Metal Hydrides for Thermal Applications in Vehicles below 0 °C. Int. J. Hydrogen Energy 2019, 44, 4878–4888. [Google Scholar] [CrossRef]
- Feldbinder Spezialfahrzeuge. Liquid Tank Semi-Trailer. Available online: https://www.feldbinder.com/en/liquid-tank-semi-trailers-for-hazardous-goods__100/ (accessed on 5 July 2024).
- Cryolor. Storage & Transport of Liquid Hydrogen. 2024. Available online: https://www.cryolor.com/sites/cryolor/files/2024-06/cryolor-lh2-brochure-01-24.pdf (accessed on 5 July 2024).
- Fuura, T.; Tsunokake, S.; Hirotani, R.; Hashimoto, T.; Akai, M.; Watanabe, S.; Enoki, H.; Akiba, E. Development of a LH2 Vehicle Tank Boil-off Gas Recovery System Using Hydrogen Storage Alloys. In Proceedings of the World Hydrogen Energy Conference, Yokohama, Japan, 27 June–2 July 2004. [Google Scholar]
- Schneider, C.; Svens, P.; Sjödin, R. Hydrogen Storage Arrangement. Swedish Patent SE 2150460 A1, 15 November 2022. [Google Scholar]
- Fujitani, S.; Nishimura, K.; Nishio, K.; Sato, K.; Yonezu, I. System-for-Storing-and-Utilizing-Hydrogen. U.S. Patent 5,728,483, 17 May 1998. [Google Scholar]
- Wang, T.C.; Morris, W.; Siegfried, J.P.; Du, J.J.; Verploegh, R.J. Liquefied Gas System with Boil-off Capture. International Patent WO 2023/225343 A2, 23 November 2023. [Google Scholar]
- Hoffmann, J. Operating Gas System for an Underwater Vehicle, Method for Operating Such an Operating Gas System and an Underwater Vehicle Having Such an Operating Gas System. U.S. Patent 9,638,372 B2, 2 May 2017. [Google Scholar]
- Friedrich, T. Einrichtung zur Druckerhöhung für Wasserstoff. German Patent DE 10 2005 004 590 A1, 10 August 2006. [Google Scholar]
- Masito, S. System for Transforming a Product. International Patent WO 2023/180142 A1, 8 September 2023. [Google Scholar]
- Kazula, S. Variable Pitot-Triebwerkseinlässe für Kommerzielle Überschallflugzeuge: Konzeptstudie Mittels eines Entwicklungsansatzes für Sichere Produkte; Springer Vieweg: Wiesbaden, Germany, 2022. [Google Scholar]
- Bürger, I.; Sourmelis Terzopoulos, V.E.; Kretschmer, C.; Kölbig, M.; Brack, C.; Linder, M. Lightweight Reactor Design by Additive Manufacturing for Preheating Applications Using Metal Hydrides. Int. J. Hydrogen Energy 2021, 46, 28686–28699. [Google Scholar] [CrossRef]
- Hahne, E.; Kallweit, J. Thermal Conductivity of Metal Hydride Materials for Storage of Hydrogen: Experimental Investigation. Int. J. Hydrogen Energy 1998, 23, 107–114. [Google Scholar] [CrossRef]
- Mendelsohn, M.H.; Gruen, D.M.; Dwight, A.E. The Effect of Aluminum Additions on the Structural and Hydrogen Absorption Properties of AB5 Alloys with Particular Reference to the LaNi5-xAlx Ternary Alloy System. J. Less-Common Met. 1979, 63, 193–207. [Google Scholar] [CrossRef]
- SAE. Definition of Commonly Used Day Types, 2023rd ed.; SAE International (AS210): Warrendale, PA, USA, 2023. [Google Scholar]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The Use of Metal Hydrides in Fuel Cell Applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef]
- Davids, M.W.; Lototskyy, M.; Malinowski, M.; van Schalkwyk, D.; Parsons, A.; Pasupathi, S.; Swanepoel, D.; van Niekerk, T. Metal hydride hydrogen storage tank for light fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 29263–29272. [Google Scholar] [CrossRef]
- Bürger, I.; Dieterich, M.; Pohlmann, C.; Röntzsch, L.; Linder, M. Standardized hydrogen storage module with high utilization factor based on metal hydride-graphite composites. J. Power Sources 2017, 342, 970–979. [Google Scholar] [CrossRef]
- Dornheim, M.; Baetcke, L.; Akiba, E.; Ares, J.-R.; Autrey, T.; Barale, J.; Baricco, M.; Brooks, K.; Chalkiadakis, N.; Charbonnier, V.; et al. Research and Development of Hydrogen Carrier Based Solutions for Hydrogen Compression and Storage. Prog. Energy 2022, 4, 042005. [Google Scholar] [CrossRef]
- SAE. Fuel Cell Vehicle Thermal Management; Energies (J3193); SAE International: Warrendale, PA, USA, 2021. [Google Scholar]
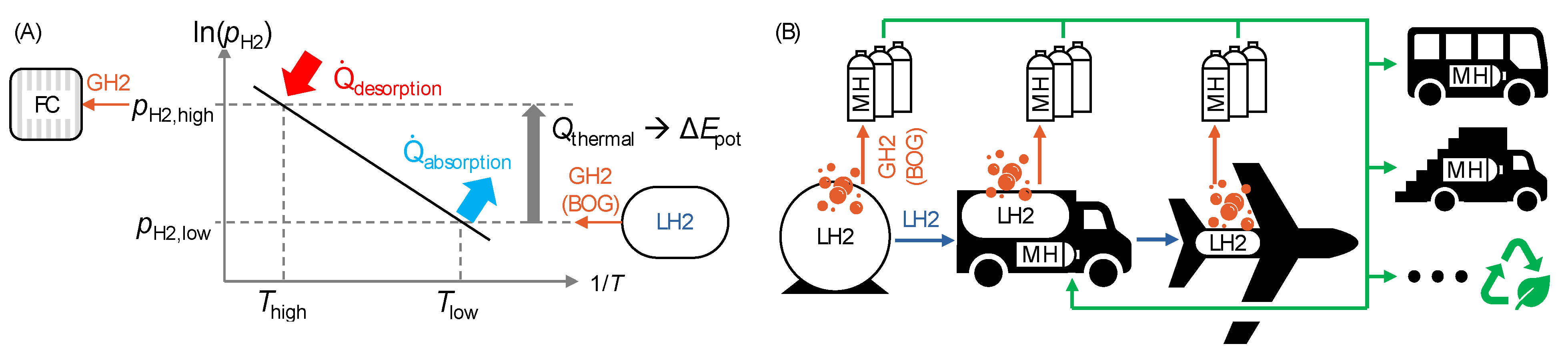
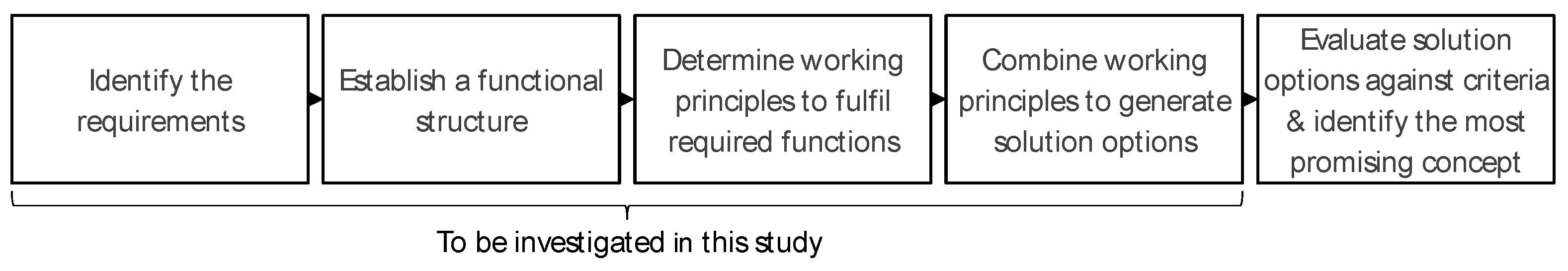
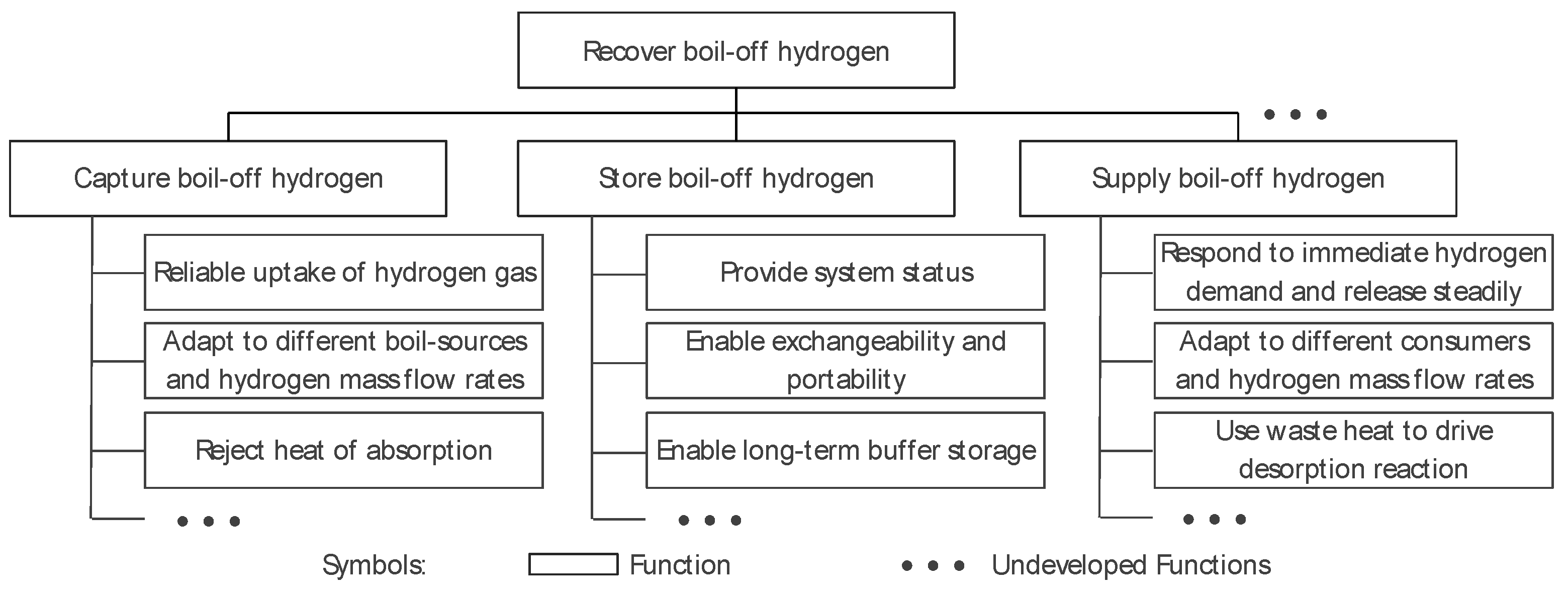
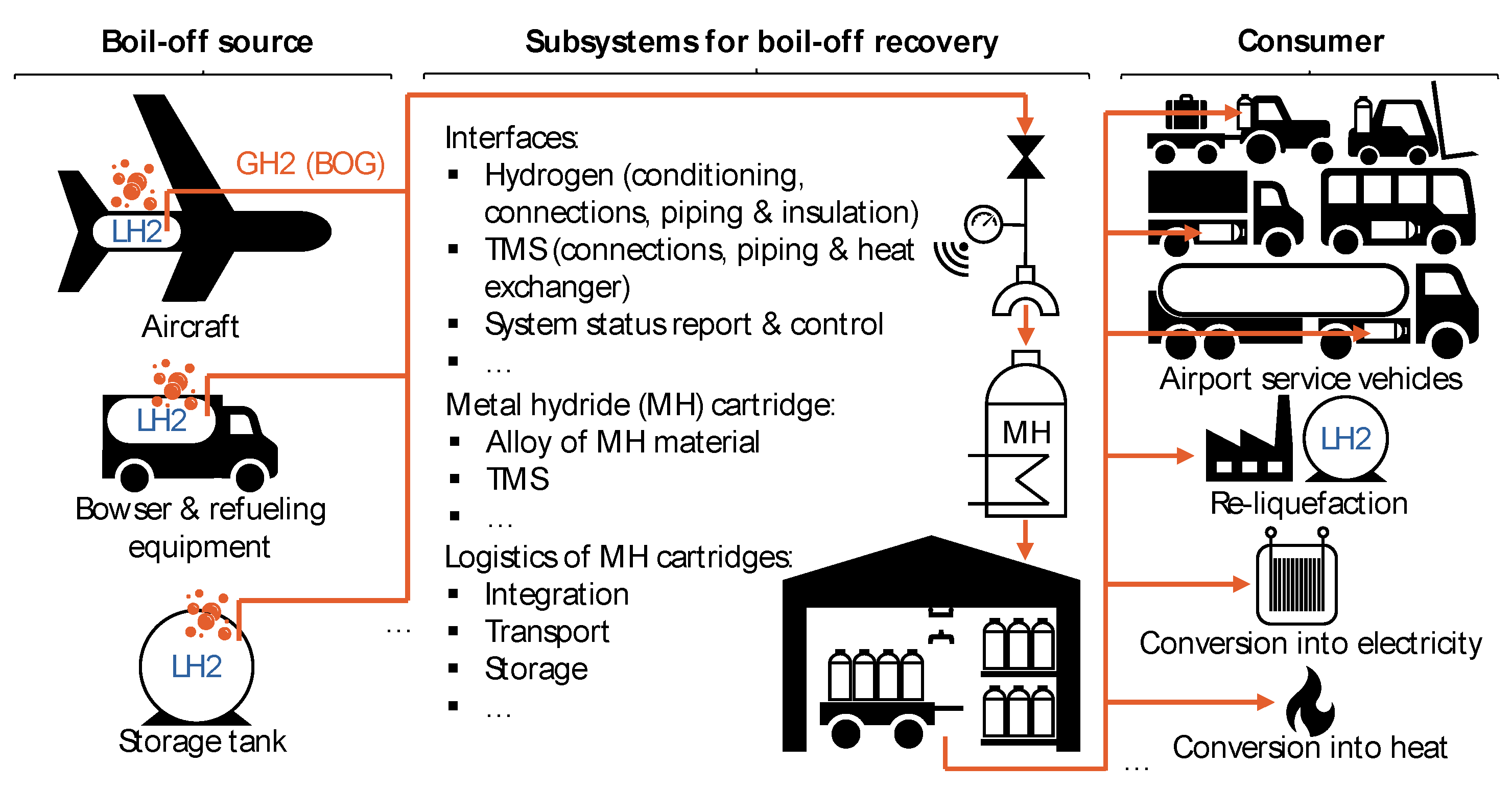
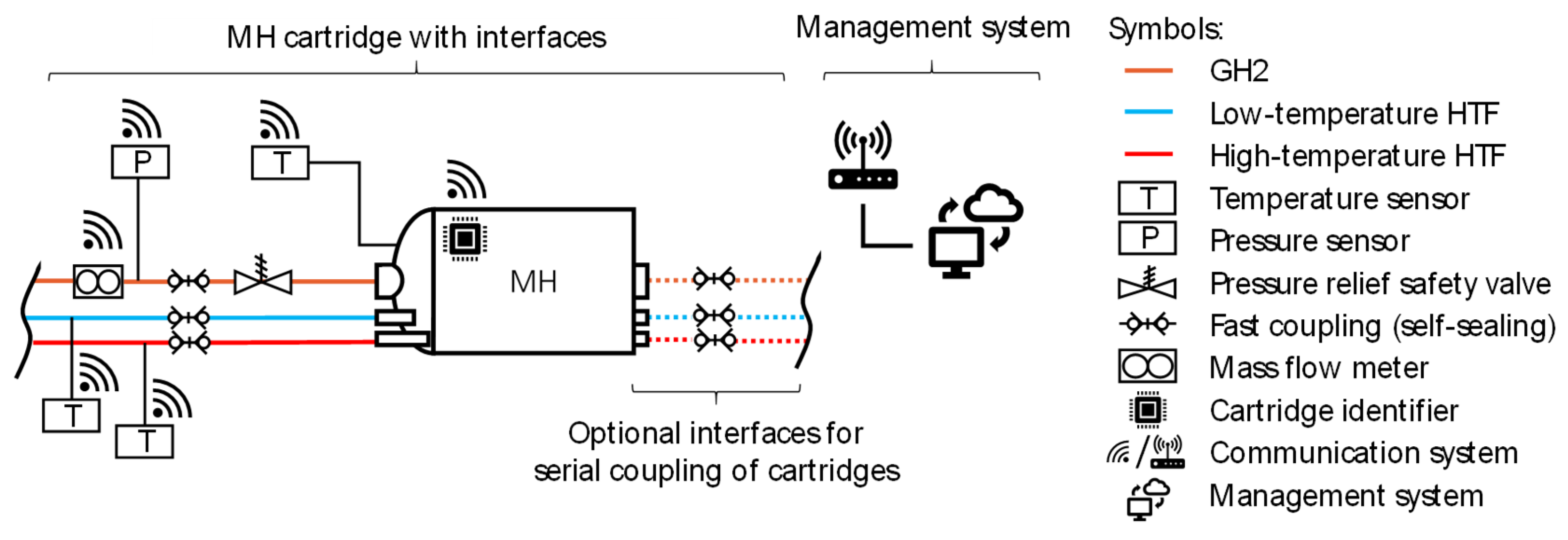
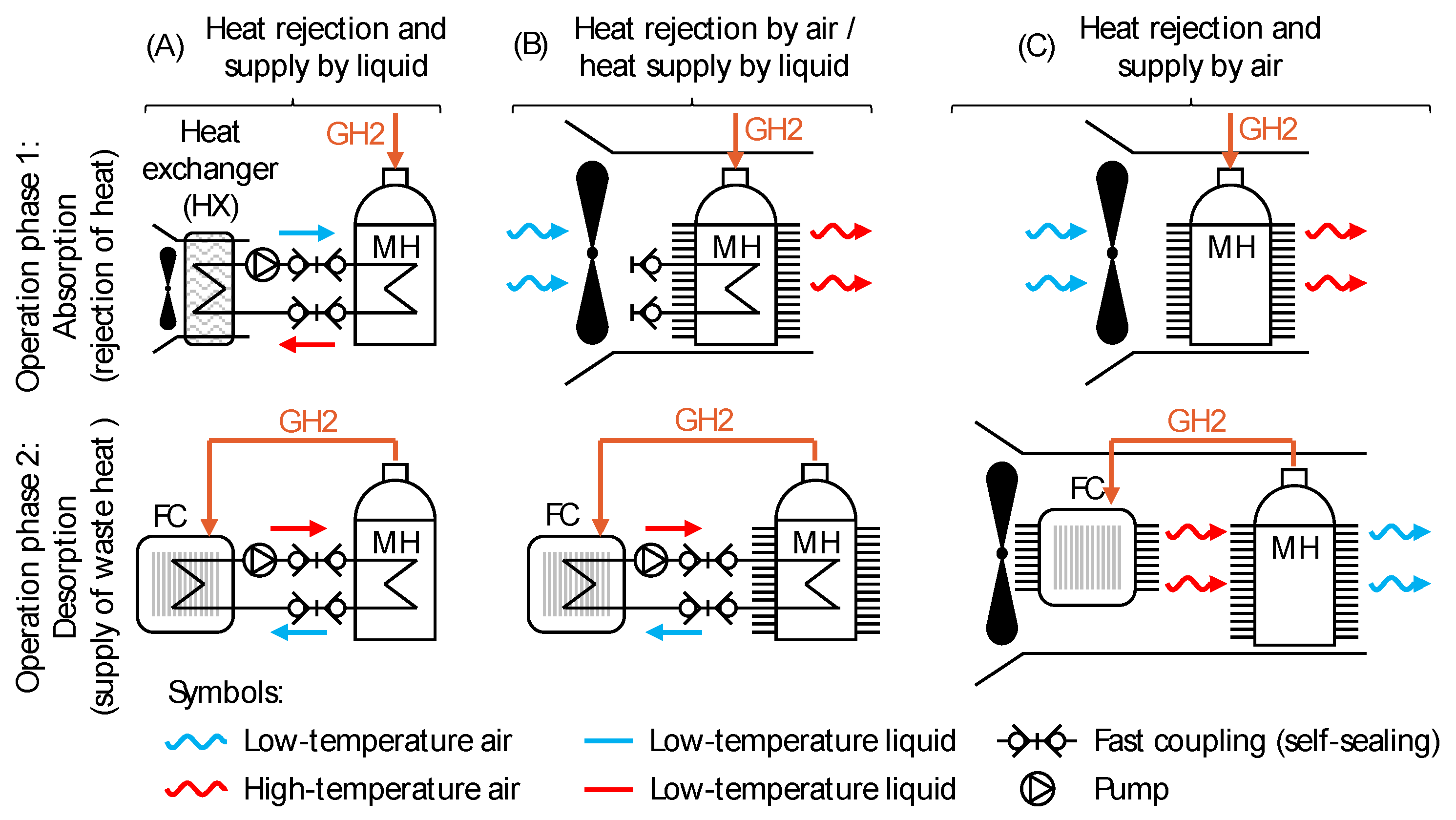
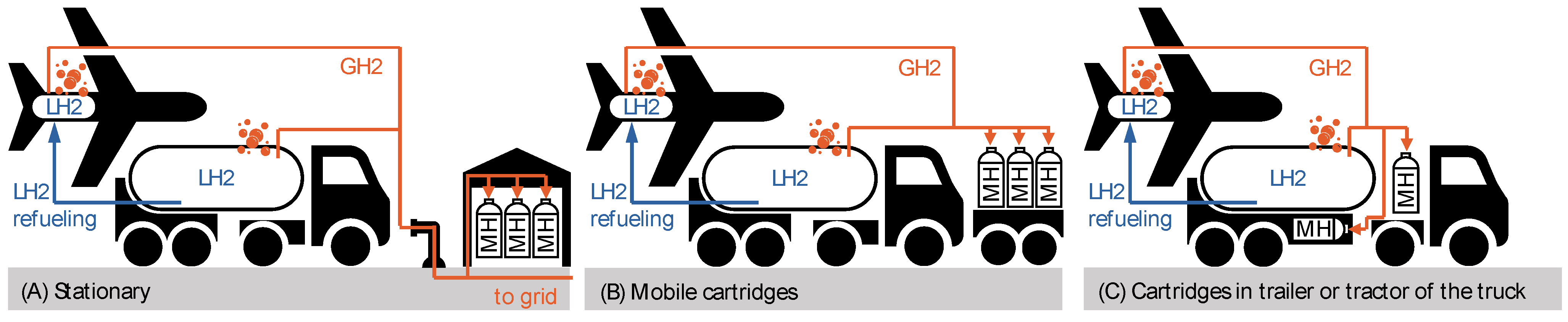
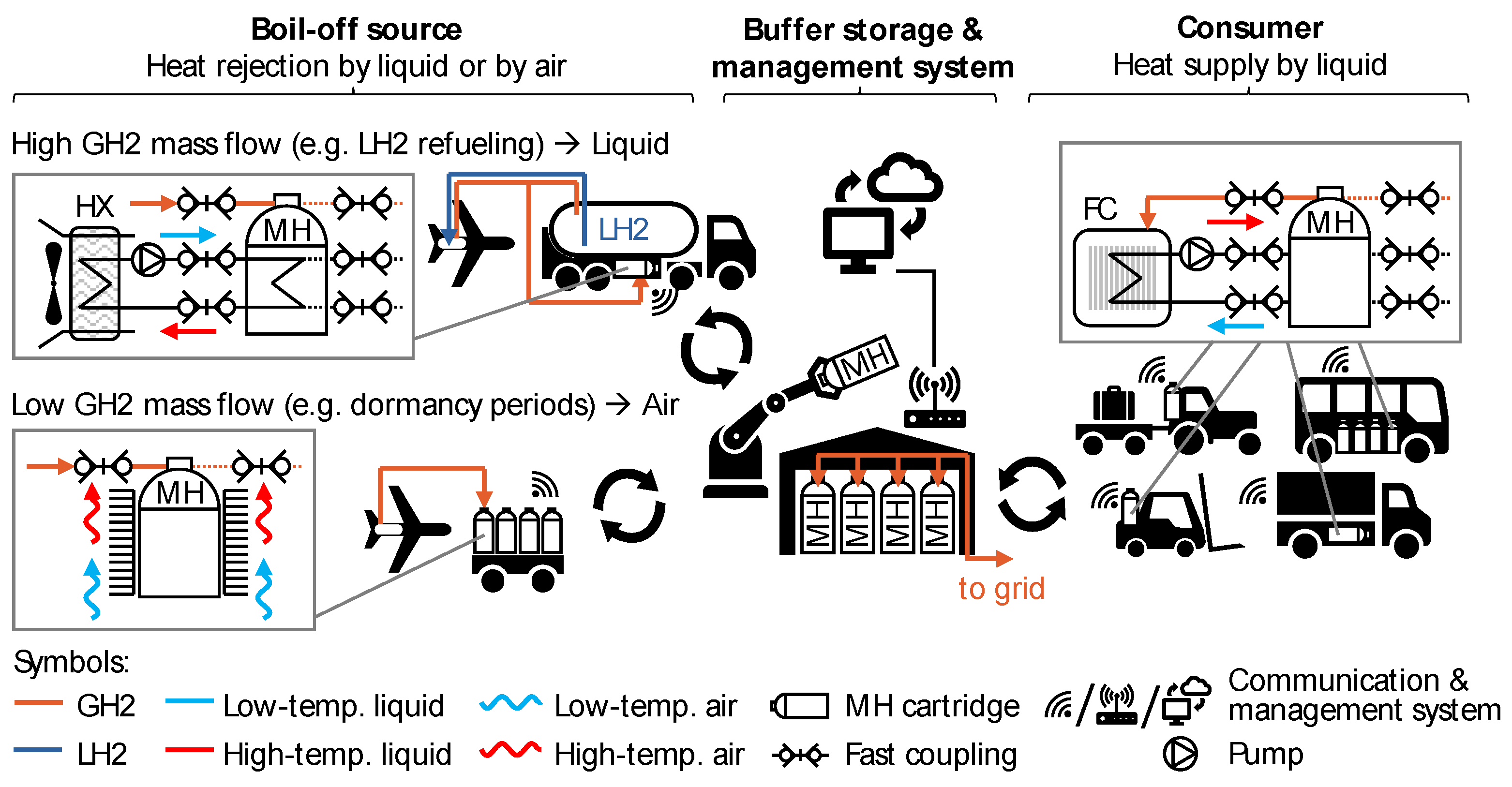
| Parameter | Value | Comments | |
|---|---|---|---|
| Storage capacity per cartridge | 2 | kgH2 | defined in [9] |
| Target absorption time | 30 | min | LH2 refueling time from [5,6,11] |
| Heat to be rejected/supplied | 18.1 | MJ/kgH2 | reaction enthalpy derived from [25] |
| Target absorption pressure | >1.2 | bar | LH2 tank pressure from [5,7] |
| Target desorption pressure | >2 | bar | FC inlet pressure from [12] |
| Temperature to reject heat | 39.4 | °C | hot day as worst case from [26] |
| Temperature to drive desorption | 80 | °C | waste heat of LT-PEMFC |
| Target capital cost of cartridge | 3000 | $/kgH2 | derived from [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franke, F.; Kazula, S. Conceptual Design of a Metal Hydride System for the Recovery of Gaseous Hydrogen Boil-Off Losses from Liquid Hydrogen Tanks. Eng. Proc. 2025, 90, 17. https://doi.org/10.3390/engproc2025090017
Franke F, Kazula S. Conceptual Design of a Metal Hydride System for the Recovery of Gaseous Hydrogen Boil-Off Losses from Liquid Hydrogen Tanks. Engineering Proceedings. 2025; 90(1):17. https://doi.org/10.3390/engproc2025090017
Chicago/Turabian StyleFranke, Florian, and Stefan Kazula. 2025. "Conceptual Design of a Metal Hydride System for the Recovery of Gaseous Hydrogen Boil-Off Losses from Liquid Hydrogen Tanks" Engineering Proceedings 90, no. 1: 17. https://doi.org/10.3390/engproc2025090017
APA StyleFranke, F., & Kazula, S. (2025). Conceptual Design of a Metal Hydride System for the Recovery of Gaseous Hydrogen Boil-Off Losses from Liquid Hydrogen Tanks. Engineering Proceedings, 90(1), 17. https://doi.org/10.3390/engproc2025090017






