Abstract
To remove nickel (II) from an aqueous solution, cellulose nanocrystals (CNCs) were modified as an adsorbent. The FTIR and SEM were used to characterise the properties of CNCs. In addition to how well they predicted reaction (adsorption capacity), the central composite design was used. The response surface model method performs well, according to statistical data. Four operational variables were studied: The initial concentration of the nickel (II) solution in mg/L, the pH, the contact period in minutes, and the adsorbent dose in g/100 mL. The removal percentage (%) was the result. The percentage removal was 98% after 178 min of contact, a starting concentration of 110 mg/L, an adsorbent dosage of 9.3 g, and an initial pH of 3.5. The R2 was 0.996, the adjusted R2 was 0.921, and the predicted R2 was 0.945. The quadratic equation was determined using central composite design. The FTIR examination revealed that the functional groups, hydroxyl groups (OH), peaked around 3300–3500 cm−1, and carboxyl groups (COOH) peaked around 1700 cm−1.
1. Introduction
The main global environmental issue of concern is heavy metal contamination. Heavy metals must be removed from wastewater since they are proven to be non-removable and detrimental to the well-being of biological systems. Among the hazardous heavy metal ions that are non-biodegradable and can be found in wastewater is nickel (II) [1], according to [2]. Nickel (II) is typically found in the byproducts of industrial (manufacturing) processes, such as mining, where it is formed from the lithosphere, painting, pigment and ceramic production, making steam electric power, and galvanisation because air can passivate nickel (II) under specific circumstances, greatly enhancing its resistance to harmful substances. On the other hand, a chrome coating is frequently applied afterwards for applications requiring galvanisation technology [3]. Nickel (II) carries a fair amount of ductility when pure, smelting, producing dyes, making batteries, as the energy transition depends on the basic material nickel (II), which is needed to transport and store renewable energy and drives lithium-ion batteries for EVs and energy storage devices, and metal finishing. Chemical oxidation/reduction, precipitation, ion exchange, adsorption, and membrane filtration are some of the methods that have been investigated to reduce the harmful effects of heavy metals on the environment and the general public to a manageable level [4].
Adsorption offers several advantages, including effectively removing a wide range of contaminants, high adsorption capacity due to large surface areas, and versatility in targeting specific pollutants. It provides environmentally friendly options using natural adsorbents, is often cost-effective with low-cost materials, and features simple design and operation. Additionally, adsorption systems are less sensitive to variations in flow rates and contaminant concentrations and can facilitate the recovery of valuable metals in specific applications [5]. As adsorbents have become more inventive and efficient, the nanoparticle demand has increased. The three-dimensional cross-linked polymers, known as superabsorbent hydrogels, could absorb and hold onto significant amounts of heavy metal ions [6]. Polymeric adsorbents have been utilised extensively for dye removal. Recently, cellulose nanocrystals have been employed extensively to create new hydrogels for specific environmental applications since they are among the most readily available and affordable polysaccharides with many hydroxyl groups [7]. With nanoparticles becoming more and more effective and innovative adsorbents, the need for their use has increased. In recent years, polymeric adsorbents have been broadly applied for the removal of dyes and heavy metal ions from water, with ion exchange, hydrogen bonding, and electrostatic interactions between heavy metal ions and the gel networks being the most prevalent mechanisms [5].
A statistical strategy for process optimisation that varies from one component at a time and involves numerous independent input factors influencing a dependent output variable is called the response surface methodology (RSM). The response is the name of the output variable. While projecting an outcome as an improved methodological approach to testing, the RSM evaluates all process parameters simultaneously [8]. One of the most important aspects of the RSM is the central composite design, which makes procedures more efficient. In a factorial or fractional factorial design, axial points (star points) and centre points make up its three constituent parts. Without needing a complete three-level factorial experiment, this design enables the estimation of a second-order (quadratic) model, incorporating nonlinear interactions between variables. CCD is especially regarded for its efficiency because it involves fewer experimental runs and yields enough information to evaluate significant effects, interactions, and response surface curvature. This makes it perfect for process optimisation and comprehending variable interactions [5].
2. Materials and Methods
The cellulose nanocrystals were hydrolysed from used paper waste (≥90%). Sodium hydroxide (≥99%) was used to increase the pH, and hydrochloric acid (32%) was used to decrease the pH. Corn starch was mixed with cellulose nanocrystals as the first step of modification. Potassium hydroxide (60%) was used to balance the pH between the CNC and starch, and ethylenediaminetetraacetic acid (98%) (EDTA) was used to modify cellulose nanocrystals into cellulose nanocrystal hydrogel. Nickel sulphate hexahydrate was used to make the aqueous solution. A nickel sulphate hexahydrate stock solution was created to form an aqueous solution of nickel (II).
2.1. Preparation of CNC-Hydrogel
A total of 250 mL of deionised water was mixed with approximately 20 mg of starch and stirred at 4 revolutions per second for 10 min at 50 °C to dissolve it. Following a 20 min infusion in 250 mL of deionised water, approximately 20 mg of CNCs was added to the dispersed starch solution. Subsequently, 60% KOH (60 g of KOH in 100 mL of distilled water) was added to the reaction flask, which was constantly stirred at 4 revolutions per second for 15 min. To start the radical polymerisation process, approximately 25 mg of ethylenediaminetetraacetic acid (EDTA) was added to the mixture, and the temperature was raised to 80 °C. After the mixture gels took about 7 min, it was rinsed with deionised water and dried at 55 °C in an oven.
2.2. Batch Adsorption
Batch experiments were conducted in 200 mL glass stoppered Erlenmeyer flask reactors that held test solutions at room temperature (25 ± 2 °C) with the concentration of Ni (II)—50 g/L and 250 g/L. The contact time was 60 min, pH was 2, and dosage was 5 g/100 mL, as shown in Table 1. In each test, 100 mL of a solution with a certain concentration of nickel (II) was added to the reactor. Using Central Composite Design, the first eight batches had a pH of 2 and 9, and a concentration of 50 mg/L and 250 mg/L, with a dosage ranging from 5 g/100 mL to 15 g/100 mL, as well as 60 min for all the batches. The remaining sample was recovered and filtered using filter paper, and the solution samples were taken. The next 8 batches consisted of pH levels of 2 and 8, and concentrations of 50 mg/L and 250 mg/L, with a dosage of 5 g/100 mL to 20 g/100 mL, and durations of 240 min. The following 14 batches consisted of pH ranging between 2 and 9, concentrations between 50 and 250 mg/L, dosages ranging between 5 g/100 mL and 15 g/100 mL, and times between 60 and 240 min. The pH was controlled using diluted 0.1 M HCl to lower the high pH and 0.1 M NaOH to increase the low pH. The contact time varied from 60 to 240 min to determine its importance. The resultant solutions were analysed using atomic absorption spectroscopy (AAS, Thermoelectric Corporation 3300).

Table 1.
The range of variables used for the model.
The removal of nickel (II) was assessed using the following equation.
Removal (%) = (CFINAL − CINITIAL/CFINAL) × 100
CFINAL is the concentration of nickel (II) at time t, and CINITIAL is the initial concentration of nickel (II) in mg/L.
3. Results and Discussion
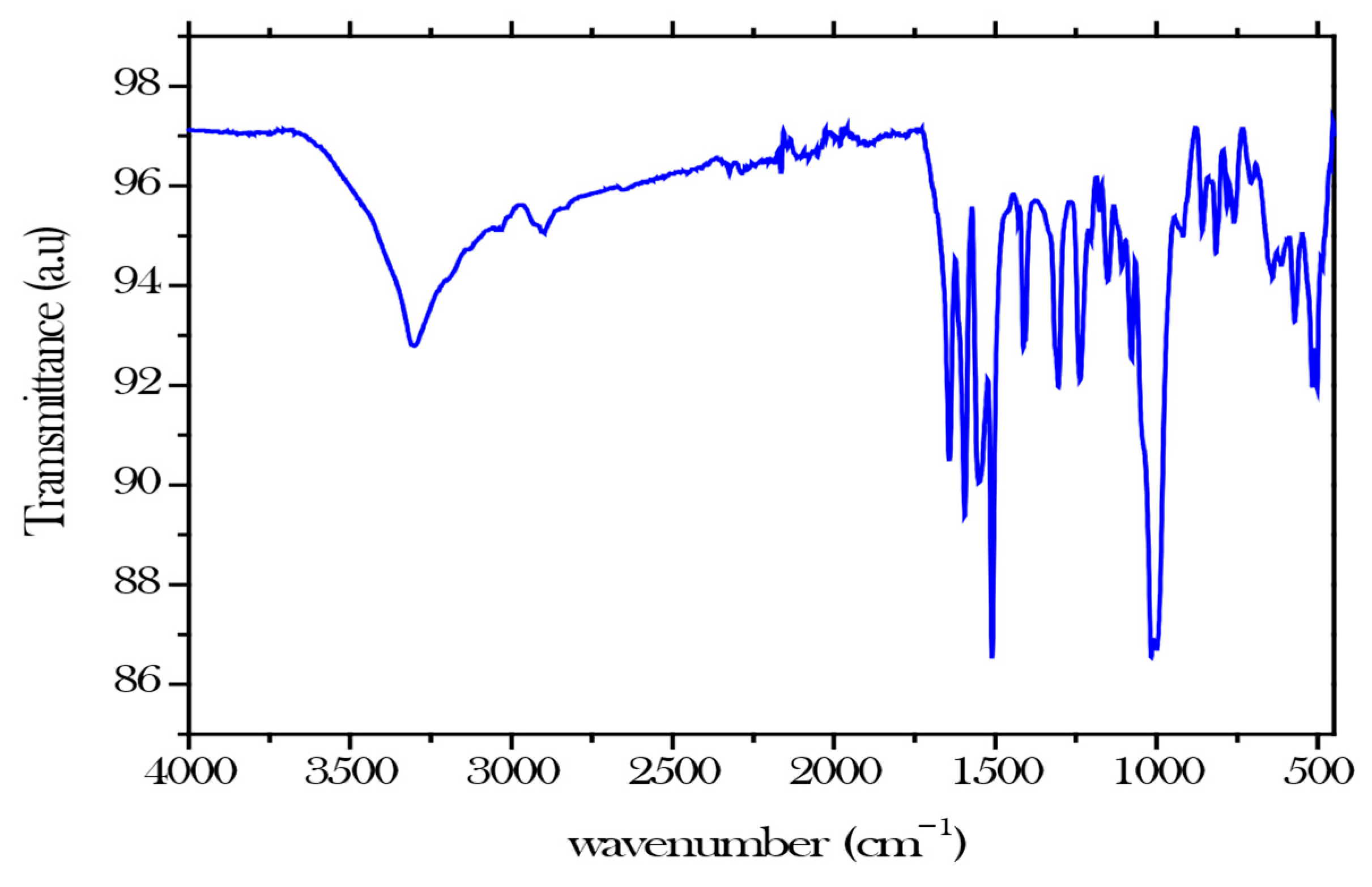
The primary objective of FTIR characterisation was to determine the chemical structure by examining the functional groups, such as carboxyl, carbonyl, and hydroxyl groups, which are crucial in achieving the primary objective of adsorbing nickel ions from aqueous solution (Figure 1). By increasing binding capacity, structural stability, and the possibility of further functionalization, the carboxyl groups (C-O) in cellulose nanocrystals greatly improve hydrogels’ efficacy in removing nickel ions from wastewater. The hydroxyl group or (O-H) typically exhibits a stretch around 3000 and 3600 , the carboxyl (COOH) and carbonyl (C=O) groups peak at 1650–1750 and 1650–1800 respectively [5].
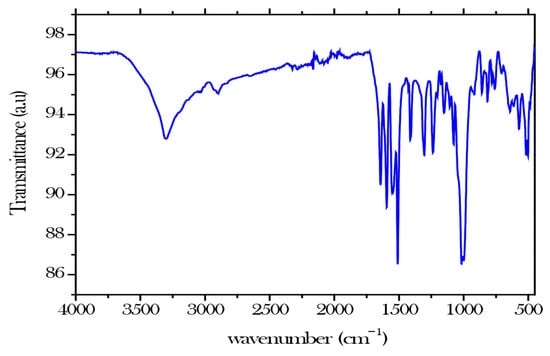
Figure 1.
FTIR for modified CNCs.
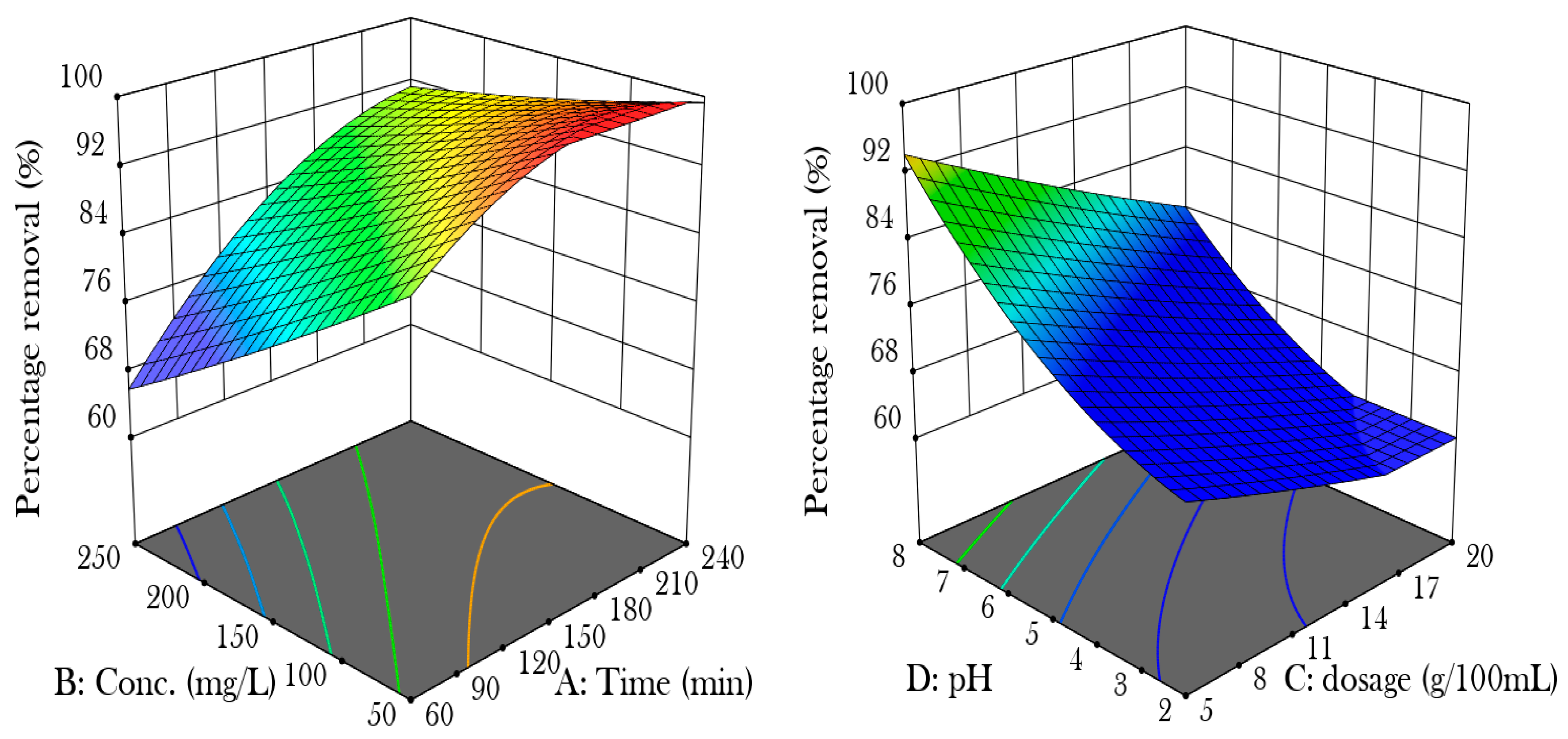
Figure 2 shows the effect of the four parameters, time (A), concentration (B), dosage (C), and pH (D) on the removal of nickel (II) from the aqueous solution. The length of the adsorption process increases its effectiveness, as the connection between nickel ions and the hydrogel becomes stronger over time due to the diffusion kinetics of the adsorption mechanism. Longer contact durations lead to more thorough engagement between the ions and the hydrogel. The initial concentration of nickel ions also impacts adsorption effectiveness, with lower concentrations promoting better cellulose nanocrystal dispersion and improved interaction with nickel ions [9]. Adsorption effectiveness in cellulose nanocrystal hydrogel remains constant after reaching a threshold due to the dynamic equilibrium between adsorption and desorption rates. The adsorbent’s active sites may become saturated, indicating that increasing the dosage beyond a threshold may not improve removal efficiency but decrease adsorption capacity returns. In acidic conditions, the presence of H+ reduces the binding capacity for metal ions, while an increase in pH decreases this competitive inhibition and increases available adsorption sites [10].
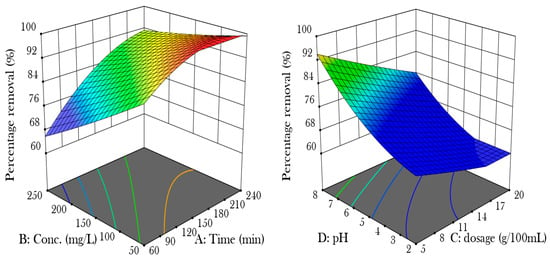
Figure 2.
Shows the effects of parameters on the adsorption efficiency.
The ANOVA in Table 2 presents the analysis results, including sources of variation, sum of squares, degrees of freedom (df), mean square, F-values, and p-values. The overall model shows a significant F-value of 12.43 with a p-value of 0.0027, indicating that the model is statistically significant and that at least one factor or interaction significantly affects the percentage removal.

Table 2.
ANOVA.
The model F-value of 12.43 implies that the model is significant. There is only a 0.27% chance that an F-value this large could occur due to noise. p-values less than 0.0500 indicate that model terms are significant. In this case, A, B, C, AC, BD, A2, D2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model. The lack-of-fit F-value of 22.39 implies that the lack of fit is significant. There is only a 0.67% chance that a lack-of-fit F-value this large could occur due to noise. Significant Terms: Factors A (time), B (concentration), C (dosage), D (pH), and interactions AC and BD have p-values less than 0.05, indicating they significantly contribute to the model. Insignificant Terms: Factors B2 (concentration squared) and C2 (dosage squared) have high p-values (>0.1), suggesting that they do not affect adsorption efficiency in any significant way.
Percentage removal = 91.38 + 8.63A − 8.11B − 2.73C + 3.87D + 4.09AB + 3.54AC − 3.07AD − 1.49BC + 6.07BD − 1.57CD − 5.21A2 + 0.5216B2 + 0.5066C2 + 4.30D2.
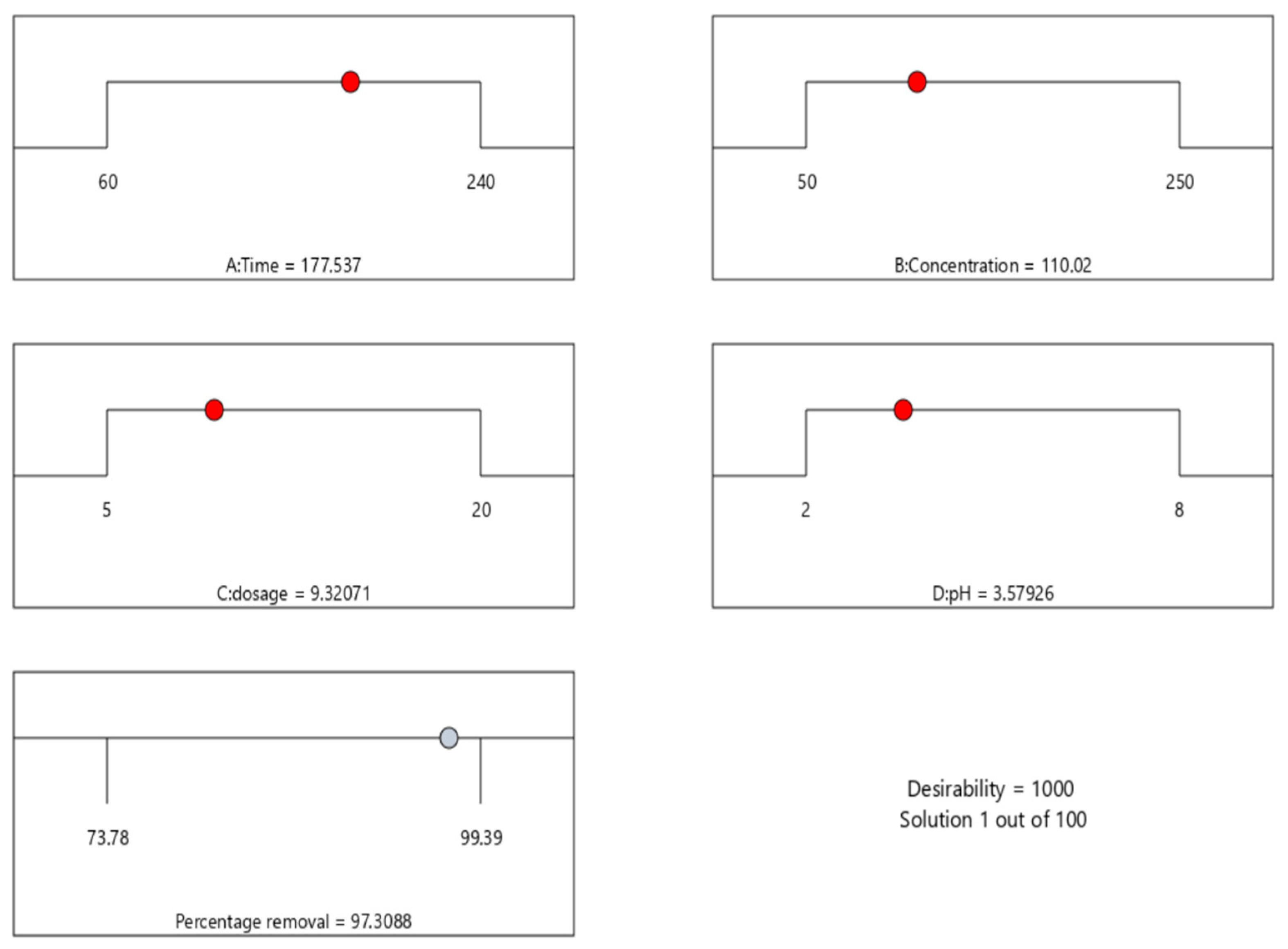
The coded factors equation can be used to predict the response for given levels of each factor. By default, the high levels of the factors are coded as +1, and the low levels are coded as −1. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients. The optimisation analysis results (Figure 3) showed that the maximum removal percentage was 97% with a pH of 3.57, a dosage of 9.32 g/100 mL, a concentration of 110.02 mg/L, and a time of 177.53 min.
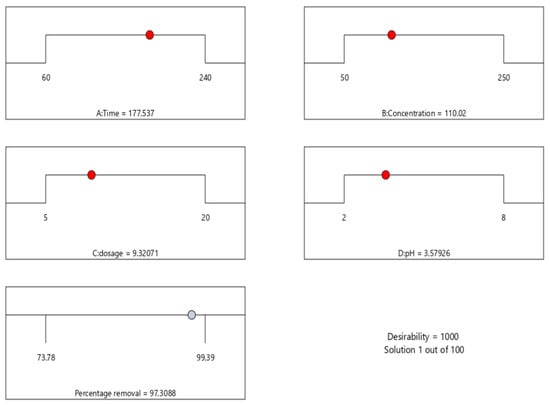
Figure 3.
Numerical optimisation for the efficient adsorption.
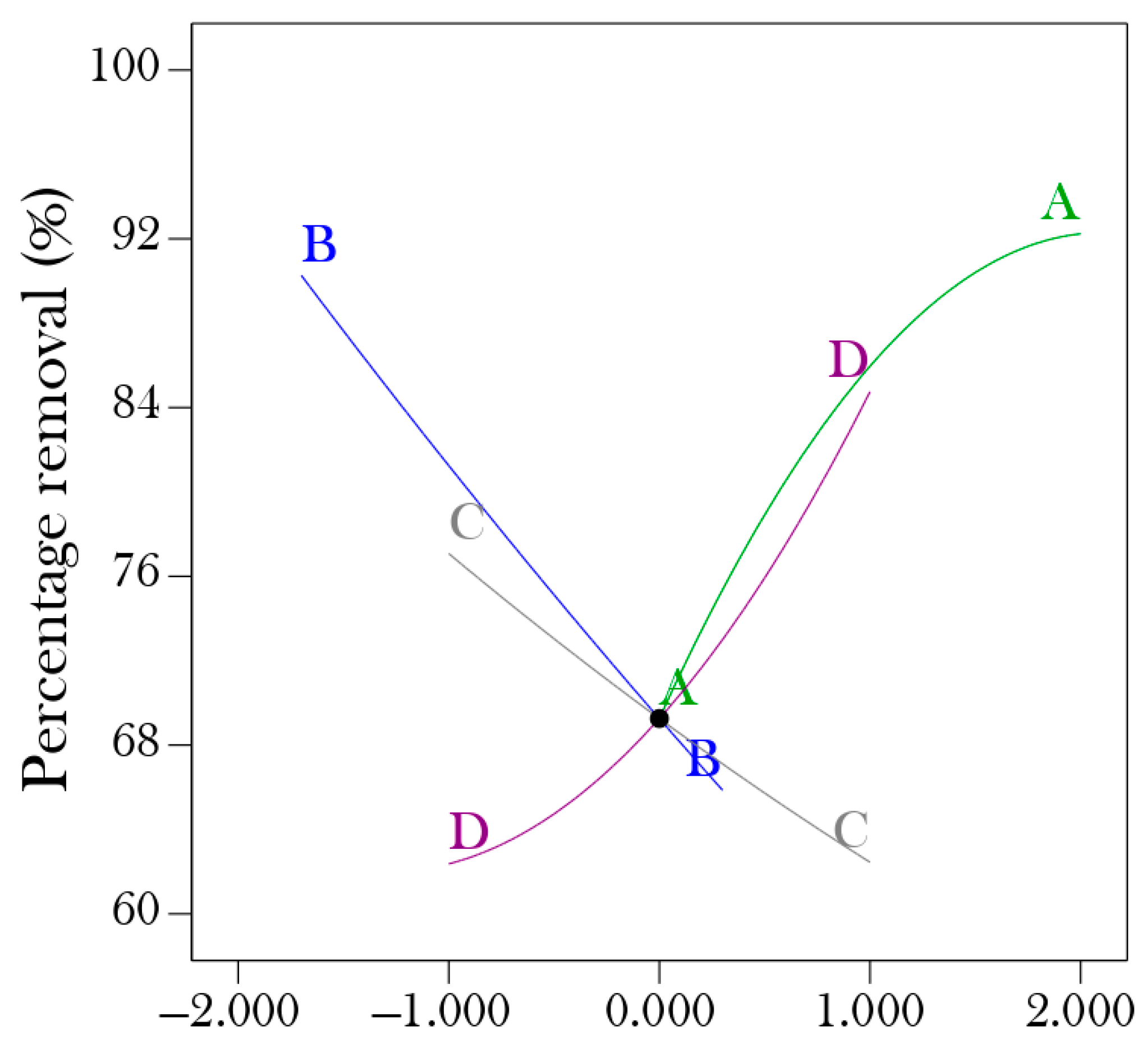
According to the perturbation plot in Figure 4, the percentage removal of nickel (II) from aqueous solution using hydrogel increases as the contact time, pH, and dosage increase, and decreases as the initial concentration increases. The outcome perfectly agrees with the variance analysis (ANOVA).
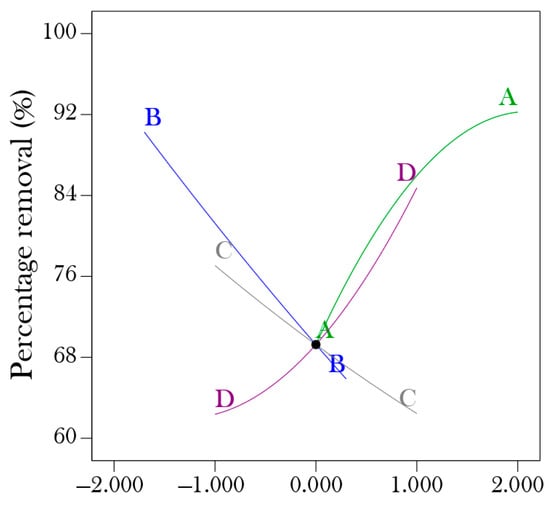
Figure 4.
Perturbation.
4. Conclusions
The FTIR and SEM were used to characterise the properties of CNCs. In addition to how well they predicted reaction (adsorption capacity), the central composite design was used. The response surface model method performs well, according to statistical data. The cellulose nanocrystal hydrogel was developed, and the hydroxyl and carboxyl groups were located at two peaks, 3000 and 3600 for the hydroxyl group and at 1650–1750 for the carboxyl group. As time increases, the adsorption efficiency rises as more time allows the nickel ions to interact with the hydrogel’s porous structure. However, after a specific period of equilibrium, desorption takes place. Higher concentrations lead to lower adsorption efficiency as more nickel ions interact with the hydrogel, while lower concentrations increase the adsorption efficiency. Dosage stabilises the adsorption efficiency, while an increase in pH enhances the adsorption efficiency due to the nature of the nickel ions having positive charges and higher levels of alkalinity (OH−). The interactions between the hydrogel’s surface area and nickel ions are maximised, increasing efficiency. The CCD effectively optimised the removal of nickel ions from aqueous solutions by identifying optimal points. At 177.537 min, the concentration is 110.02 ppm, the dosage is 9.321 g/100 mL, and the pH is 3.58, which are the optimal points, producing an efficiency removal of 97.31%. The R2 was 0.996, the adjusted R2 was 0.921, and the predicted R2 was 0.945. The quadratic equation was determined. The ANOVA stated that A, B, C, AC, BD, A2, and D2 are significant model terms.
Author Contributions
Conceptualisation, L.N. and M.B.; methodology, L.N., M.B. and T.S.; software, M.B.; validation, L.N., M.B. and T.S.; formal analysis, L.N., M.B. and T.S.; investigation, L.N., M.B. and T.S.; resources, L.N., M.B. and T.S.; data curation, L.N. and M.B.; writing—original draft preparation, L.N. and M.B.; writing—review and editing, M.B. and T.S.; visualisation, L.N. and M.B.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The author would like to thank the Vaal University of Technology’s Department of Chemical & Metallurgical Engineering for providing continuous operating facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CNC | Cellulose nanocrystals |
| CNC-H | Cellulose nanocrystals hydrogel |
| FTIR | Fourier transform infrared spectroscopy |
| RSM | Response surface method |
| CCD | Central composite design |
References
- Es-Sahbany, H.; Berradi, M.; Nkhili, S.; Hsissou, R.; Allaoui, M.; Loutfi, M.; Bassir, D.; Belfaquir, M.; El Youbi, M.S. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today Proc. 2019, 13, 866–875. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2021, 28, 2572–2599. [Google Scholar] [CrossRef] [PubMed]
- Kabuba, J.; Banza, M. Modification of clinoptilolite with dialkylphosphinic acid for the selective removal of cobalt (II) and nickel (II) from hydrometallurgical effluent. Can. J. Chem. Eng. 2021, 99, S168–S178. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A review on cellulose nanocrystals as promising biocompounds for the synthesis of nanocomposite hydrogels. Carbohydr. Polym. 2019, 216, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Banza, M.; Seodigeng, T.; Rutto, H. Comparison Study of ANFIS, ANN, and RSM and Mechanistic Modeling for Chromium(VI) Removal Using Modified Cellulose Nanocrystals–Sodium Alginate (CNC–Alg). Arab. J. Sci. Eng. 2023, 48, 16067–16085. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Danial, W.H.; Majid, Z.A.; Muhid, M.N.M.; Triwahyono, S.; Bakar, M.B.; Ramli, Z. The reuse of wastepaper for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2015, 118, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, K.O.; Ahmed, N.A.; Madyira, D.M.; Adebayo, A.O.; Ogunkunle, O.; Adeleke, O. Performance evaluation of ANFIS and RSM modeling in predicting biogas and methane yields from Arachis hypogea shells pretreated with size reduction. Renew. Energy 2022, 189, 288–303. [Google Scholar] [CrossRef]
- Ayoola, A.A.; Hymore, F.K.; Omonhinmin, C.A.; Babalola, P.O.; Fayomi, O.S.I.; Olawole, O.C.; Olawepo, A.V.; Babalola, A. Response surface methodology and artificial neural network analysis of crude palm kernel oil biodiesel production. Chem. Data Collect. 2020, 28, 100478. [Google Scholar] [CrossRef]
- Kabuba, J.; Banza, M. Results in engineering ion-exchange process for the removal of Ni(II) and Co(II) from wastewater using modified clinoptilolite: Modeling by response surface methodology and artificial neural network. J. Results Eng. 2020, 8, 100189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).