1. Introduction
Electrochemistry is a discipline that studies chemical reactions involving electron transfer between an analyte (substance under study) and the surface of an electrode, as well as the properties of interfaces and the general properties of electrolytes (electrically charged ions in a solution). Electrochemical reactions are the basis for several areas of research, including energy conversion and storage, corrosion, sensor, and biosensor applications. To enable the study of these reactions in a wide range of applications, it has been necessary to develop several electrochemical techniques such as cyclic voltammetry, chronoamperometry, chronopotentiometry, scanning electrochemical microscopy, and electrochemical impedance spectroscopy (EIS) [
1].
EIS is a characterization technique that allows the study of properties at electrochemical interfaces, such as metal/solution and electrode/solution interfaces, where an alternating excitation voltage is applied, and its response is measured relative to the impedance of the system under study. EIS provides information about the physical and chemical properties of the system by analyzing impedance (Z, measured in ohms, Ω), frequency (f, measured in hertz, Hz), and phase (θ, measured in degrees, °) parameters. Impedance measures a system’s opposition to electric current, depending on resistance (R, measured in ohms, Ω) and reactance (X, measured in ohms, Ω). Frequency is the number of cycles an alternating signal completes per unit time, typically ranging from 0.01 Hz to 10 megahertz (MHz). Phase is the angle between the applied and measured signals, reflecting the time lag between current and voltage [
2].
Pesticides are widely used chemicals, particularly in agriculture, to control insect pests in crops such as cotton, corn, wheat, fruits, and vegetables. However, their indiscriminate and excessive use has led to the contamination of water sources and significant environmental and health impacts. This is particularly concerning for organophosphate pesticides, which can cause acute or chronic poisoning, endocrine disruption, neurotoxicity, cancer, and genetic damage. Chlorpyrifos is one of the most commonly used organophosphorus pesticides (OPs) in agriculture and is a major contributor to the toxicity affecting aquatic life, soil, and food, impacting biodiversity and natural resources as it contaminates waterways [
3]. Chlorpyrifos is a neurotoxic compound that irreversibly inhibits acetylcholinesterase (AChE), an essential enzyme for the central nervous system (CNS) function in humans and insects. When AChE is immobilized on a screen-printed electrode (SPE), the interaction between the substrate and the electrode surface produces an electroactive species, releasing electrons that can be measured by the developed electronic system [
4].
To address concerns regarding pesticide contamination, it is essential to develop analytical methods for detecting and quantifying pesticides in environmental and biological samples. Available techniques include gas chromatography coupled to mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), infrared spectroscopy (IR), ultraviolet–visible spectroscopy (UV-Vis), and fluorescence spectroscopy (FL). However, these techniques often require expensive, complex, and bulky equipment, along with extensive sample preparation involving organic solvents and chemical reagents.
In this context, EIS emerges as a promising alternative for chlorpyrifos analysis due to its non-destructive, simple, fast, and sensitive nature. This technique enables the evaluation of contaminant interactions with biosensors, contributing to the development of more effective detection methods and reducing environmental and health risks associated with pesticide contamination.
Biosensors are analytical devices that leverage the sensitivity and selectivity of a bioreceptor, which is attached to a transducer. The transducer converts biochemical or physicochemical properties into measurable signals resulting from the interaction between the bioreceptor and the target analyte [
3]. Amperometric biosensors measure current changes at the working electrode (WE, measured in amperes, A) due to oxidation or reduction reactions. When selecting a detection method, key factors include cost, sensitivity, reliability, and speed. Electrochemical analysis offers a cost-effective approach to quantifying chemicals and detecting material property changes with high selectivity and sensitivity [
4].
Electrochemical techniques require electrochemical sensors composed of two or three electrodes: a working electrode (WE), a reference electrode (RE), and a counter electrode (CE) (auxiliary electrode), along with electronic instrumentation to collect, process, and store experimental data. While conventional electronic instrumentation is often bulky and expensive, recent advances in electronics allow the miniaturization of these devices, facilitating their use in field tests [
5].
In this study, we propose the development and application of a portable system based on modular and commercial electronic components. The system integrates a PalmSens EmStatPico Module for electrochemical measurements, a Raspberry Pi Zero 2W (RPi Zero 2W) (Raspberry Pi Foundation, Cambridge, England, UK) [
6] as a wireless data server, and a solar charge manager module powered by a lithium-ion battery and a 5 V, 1 A solar panel. Python-based software was developed to control the routines executed by the EmStatPico Module.
This study aims to demonstrate the feasibility, simplicity, and analytical sensitivity of the developed portable system for detecting chlorpyrifos in aqueous samples using EIS. The pesticide is detected using an enzymatic amperometric biosensor in sterile water samples prepared in the laboratory and deposited on the surface of an SPE.
2. Materials and Methods
This research followed a structured approach consisting of three main stages: variable determination, data collection, and system implementation. These stages are described in the following subsections.
2.1. Instrumentation and Experimental Setup
Electrochemical measurements were performed using a PalmSens EmStatPico Module configured as a potentiostat/galvanostat for electrochemical impedance spectroscopy (EIS). The EmStatPico is a compact and portable module that operates either on a rechargeable battery or via USB. Key specifications include a 16-bit resolution, a voltage range of −1.65 V to 1.65 V, a current range of −100 µA to 100 µA, internal impedance below 10 Ω, and a maximum frequency of 200 kHz [
7].
The module was connected to a Raspberry Pi Zero 2W using four wires (5 V, GND, TX, RX). The RPi Zero 2W is a single-board computer featuring a 1 GHz ARM Cortex-A53 processor, 512 MB RAM, and integrated Wi-Fi and Bluetooth. It was configured as a web server for wireless access to measurement data.
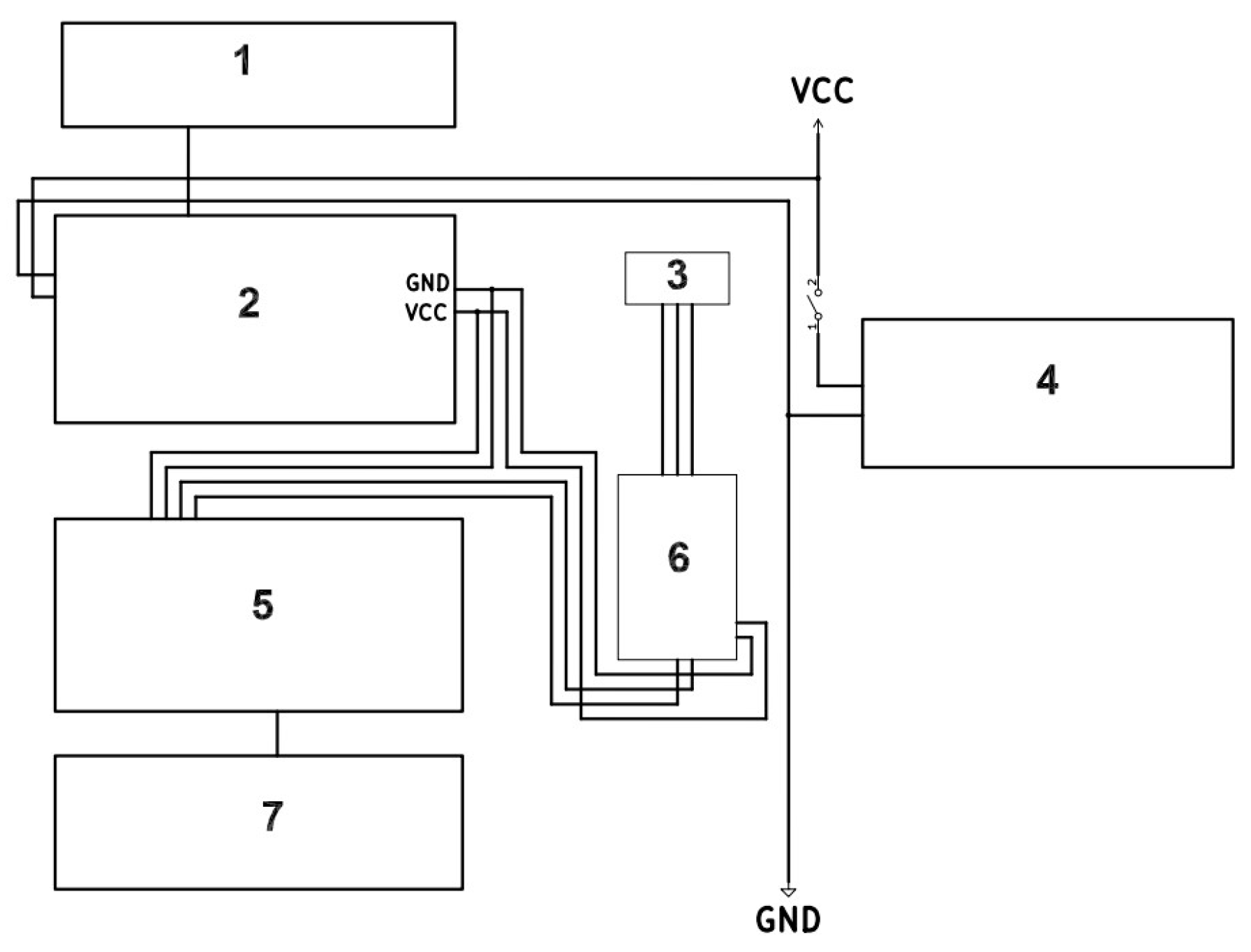
The complete system, including the photovoltaic module, energy management components, and signal interface, is illustrated in
Figure 1.
A Python-based control software was developed using PalmSens MethodSCRIPT libraries [
8], enabling automated execution of EIS routines and data visualization. The software communicates with the EmStatPico via serial text-based commands. A pseudocode overview is shown in
Table 1.
The EmStatPico Module was connected to a Raspberry Pi Zero 2W (RPi Zero 2W) using only four wires: 5 V, GND, TX, and RX. The RPi Zero 2W is a single-board computer (SBC) equipped with a 1 GHz ARM Cortex-A53 processor, 512 megabytes (MB) of random-access memory (RAM), and Wi-Fi and Bluetooth connectivity [
6]. The RPi Zero 2W was configured as a web server to enable access to data generated by the EmStatPico Module from any internet-connected device.
Python-based software was developed to control the measurement routines executed by the EmStatPico Module, as detailed in the pseudocode shown in
Table 1. The routines were based on MethodSCRIPT libraries, which provide a set of functions for communication with the EmStatPico Module via text-based commands [
7]. These libraries simplify the programming and customization of electrochemical measurements and facilitate the acquisition and processing of the obtained data. The developed software utilizes MethodSCRIPT libraries for Python [
8], which are compatible with the RPi Zero 2W running Raspberry Pi OS.
2.2. Electrochemical Biosensor and Cell Configuration
The sensing component consisted of a commercial amperometric biosensor (AC1.AChE, BVT Technologies [
9]) integrating a three-electrode system printed on a ceramic substrate. The setup included the following:
A working electrode (WE) coated with acetylcholinesterase (AChE) immobilized on a carbon layer.
A counter electrode (CE) made of carbon (also called auxiliary electrode).
A reference electrode (RE) composed of silver/silver chloride (Ag/AgCl).
The electrodes were screen-printed (SPE), a format that offers a reproducible and cost-effective platform for biosensing. The device featured an active area of 4 mm2 and a sensitivity of 0.1 nA/nM for acetylcholine (ACh). The biosensor was connected to the EmStatPico module via a custom interface. A 5 mL droplet of the water sample was applied directly onto the SPE surface. During measurements, the electrochemical cell was maintained at room temperature (~20 °C) and placed on an anti-vibration surface to minimize signal noise.
2.3. Sample Preparation and Experimental Conditions
Nine aqueous samples were prepared using distilled water and different concentrations of LorsbanTM 2.5% DP (2.5% chlorpyrifos). Sample concentrations were adjusted by adding 0.088 g, 0.029 g, and 0.01 g of Lorsban to 5 mL of distilled water. Each concentration was tested in triplicate, and control samples (distilled water only) were also analyzed.
Since chlorpyrifos is a known inhibitor of acetylcholinesterase (AChE), its presence can significantly affect the biosensor’s response.
Samples were homogenized using magnetic stirring for 10 min at 20 °C. An analytical balance (Kern ABJ-NM/ABS-N) was used to ensure precision during sample preparation.
2.4. Electrochemical Impedance Spectroscopy (EIS) Analysis
EIS measurements were performed over a frequency range of 20 Hz to 200 kHz, using a sinusoidal excitation of 15 mV superimposed on the open circuit potential (OCP). The impedance magnitude (|Z|) and phase angle (θ) were recorded at each frequency point. For analytical consistency, the evaluation of sensor performance focused on the 100 Hz to 10 kHz range. This interval was selected because it balances signal stability, sensitivity to interfacial electrochemical effects, and noise reduction. Similar ranges have been recommended in the literature for AChE-based impedance biosensors, where frequencies below 100 Hz often introduce instability and delays, while mid-range frequencies enhance sensitivity to variations in double-layer structure and charge transfer resistance [
10,
11,
12].
The data were processed and visualized using a custom Python application developed for the EmStatPico Module. Bode plots were generated to analyze the biosensor’s frequency response and its correlation with chlorpyrifos concentration.
3. Results
The system successfully recorded impedance and phase responses for nine water samples containing varying concentrations of chlorpyrifos, along with two control samples prepared using distilled water. Frequency-dependent behavior was visualized through Bode plots, which provided a comprehensive representation of both impedance magnitude (|Z|) and phase angle (θ) across the measured frequency spectrum.
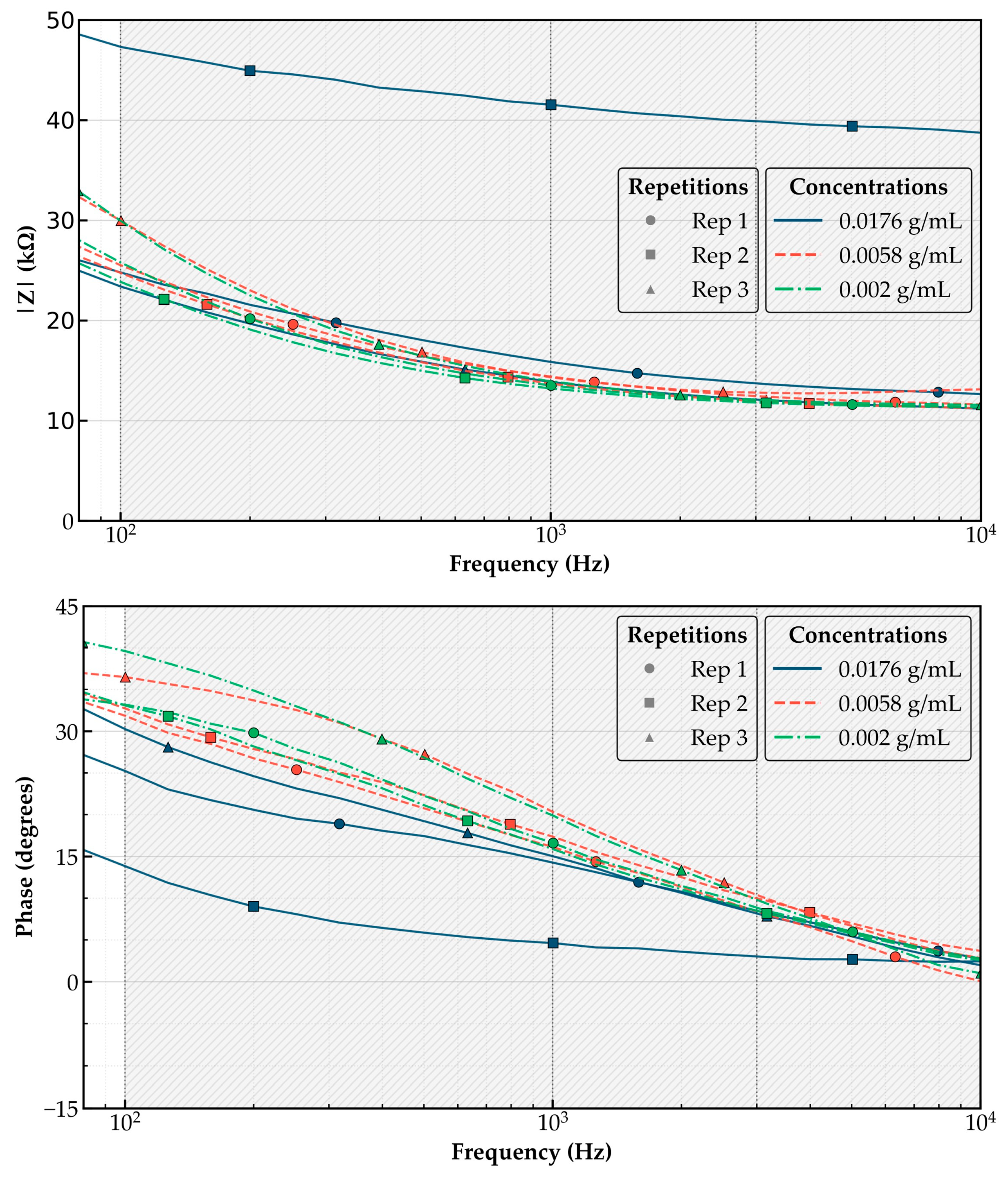
As shown in
Figure 2, the impedance magnitude (|Z|) consistently decreased with increasing frequency, a typical characteristic of capacitive electrochemical systems. More importantly, chlorpyrifos-treated samples exhibited elevated impedance values relative to lower concentrations, indicating a concentration-dependent effect. This response is attributed to the inhibition of enzymatic activity by chlorpyrifos, which alters charge transfer dynamics and modifies the interfacial electrochemical environment, which alters the local electrochemical environment. The effect is particularly pronounced in the 100–1000 Hz region, where interfacial charge processes dominate, as evidenced by the steep slope of the |Z| curves and clear separation among concentration groups.
Additionally, the phase angle (θ) decreased with increasing chlorpyrifos levels, revealing a shift in the system’s electrochemical response toward a more resistive and less capacitive behavior. This reinforces the hypothesis that pesticide exposure disrupts the electrode–electrolyte interface by inhibiting AChE activity and modifying charge distribution and polarization mechanisms.
For analytical consistency, impedance trends were evaluated specifically within the 100 Hz to 10 kHz range. This interval was selected based on both experimental evidence and recommendations from prior EIS-based biosensing studies, which indicate that it offers an optimal balance between signal fidelity, sensitivity to interfacial phenomena, and immunity to capacitive noise at higher frequencies or instability at lower ones [
11,
12,
13]. The observed trend of increasing |Z| with analyte concentration was most clearly distinguishable within this frequency window, reinforcing the sensor’s capacity to differentiate pesticide levels.
Impedance responses exhibited moderate variability between replicates, primarily due to biosensor heterogeneity or instrumental fluctuations. However, as shown in
Table 2 and
Figure 2, the sensor consistently distinguished between the three tested concentrations, particularly within the 100–1000 Hz range, where interfacial processes are most pronounced. The average |Z| values at 100 Hz were 12.83, 15.67, and 19.46 kΩ for 0.002, 0.0058, and 0.0176 g/mL, respectively. The standard deviations remained relatively low for the 0.002 and 0.0058 g/mL groups (0.68 and 0.52 kΩ at 100 Hz, respectively), supporting the reproducibility of the measurements at lower analyte levels. Although the 0.0176 g/mL group exhibited greater variability (1.27 kΩ at 100 Hz and up to ~5.7 kΩ at 10 kHz), this reflects the sensor’s increased sensitivity at higher analyte concentrations. Despite this dispersion, the trend of rising impedance with concentration was preserved at all measured frequencies. These results confirm the sensor’s capacity to differentiate chlorpyrifos levels with statistically meaningful resolution, even under moderate experimental variability.
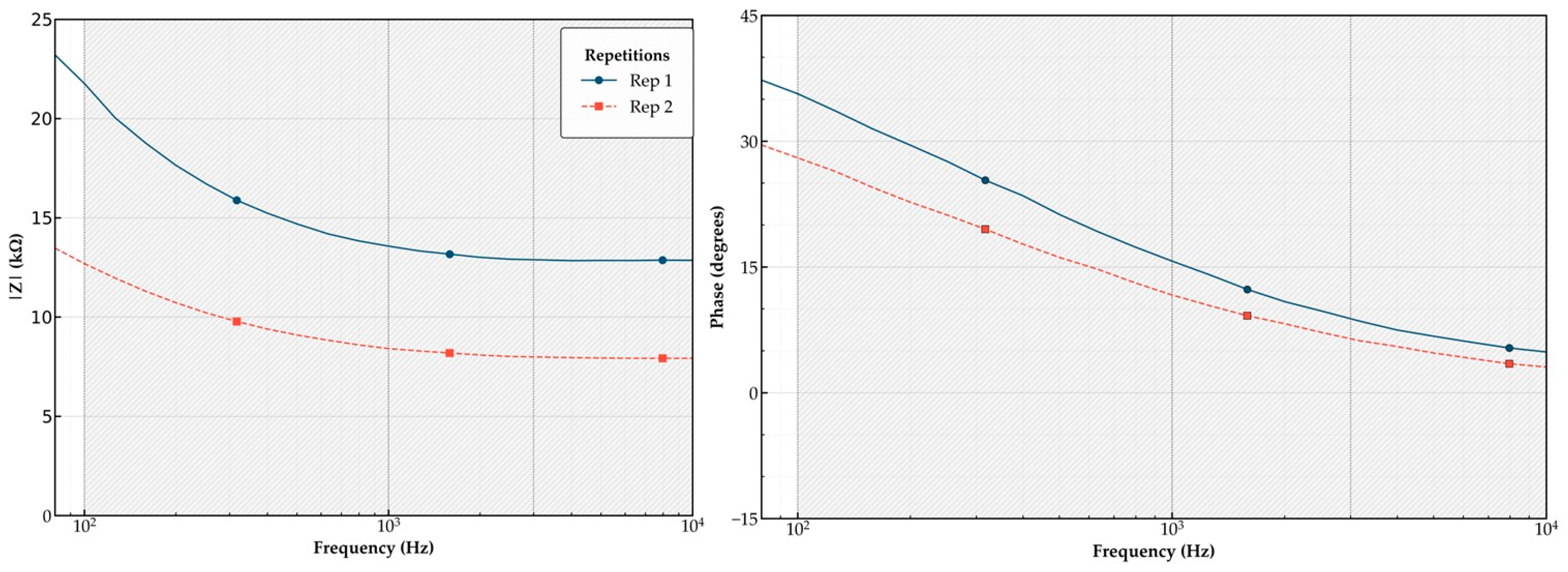
In the absence of chlorpyrifos, the AChE enzyme remains catalytically active, promoting charge transfer and resulting in lower conductivity and higher impedance. This behavior is evident in the control sample’s Bode plots (
Figure 3), where |Z| exceeds 23 kΩ at 100 Hz, and the phase angle increases to 35°. These observations validate the inverse relationship between pesticide inhibition, ionic strength, and electrochemical response.
Table 3 summarizes the measured electrical conductivity (σ) for each chlorpyrifos concentration across three replicates. As expected, the control group showed the lowest value (3.05 µS/cm), consistent with its high impedance (
Figure 3). Although conductivity increased with analyte concentration, the relationship was not strictly linear. For instance, the 0.0058 g/mL group overlapped with the 0.002 g/mL values (both around 44.5 µS/cm). These findings suggest that ionic strength is influenced by factors such as sample handling and sensor surface interactions, not solely by chlorpyrifos levels.
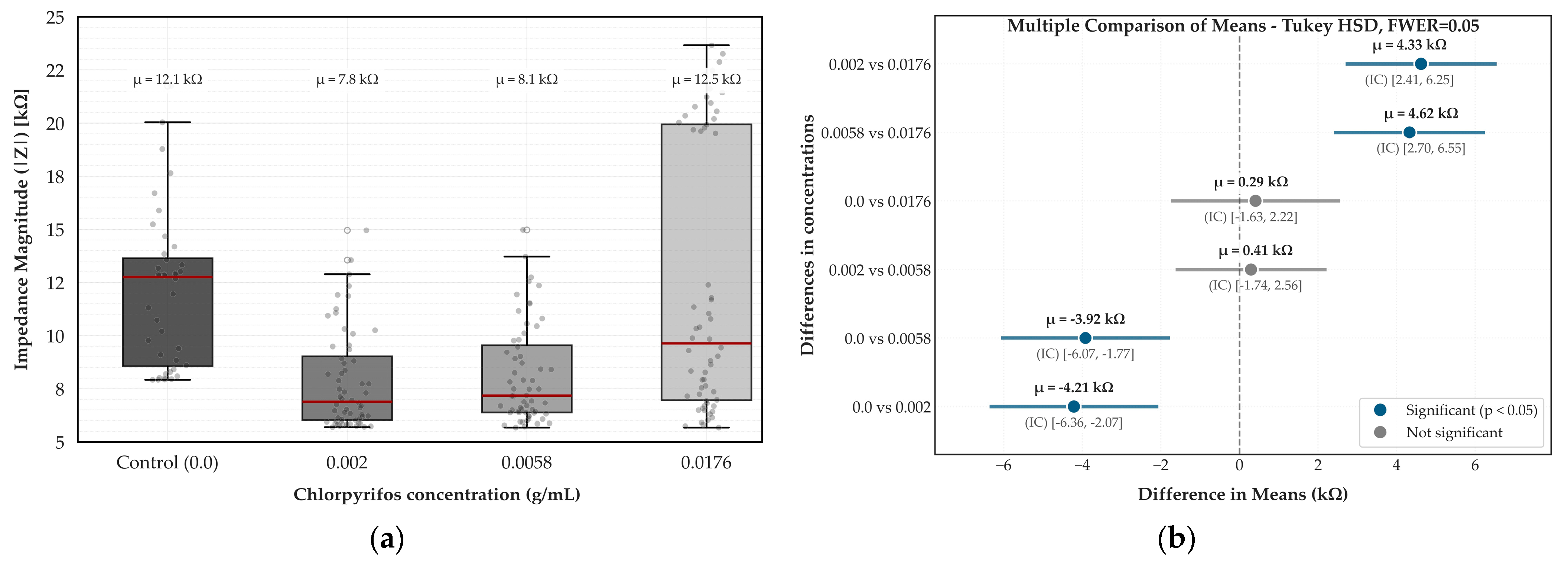
To statistically validate the system’s detection capabilities, an ANOVA was performed on impedance values within the 100 Hz to 10 kHz range, previously identified as the sensor’s most sensitive window (
Figure 2). As illustrated in
Figure 4a, the box plots reveal impedance magnitude (|Z|) differed significantly across concentration groups (F = 20.59,
p < 0.001). The control group (0.0 g/mL) showed significantly higher impedance than both 0.002 and 0.0058 g/mL (mean differences above 4 kΩ,
p < 0.001), confirming the system’s ability to detect low chlorpyrifos concentrations. In contrast,
Figure 4b illustrates that the difference between the control and 0.0176 g/mL was not statistically significant (
p = 0.961), suggesting a return of the signal to baseline levels at higher concentrations.
This biphasic response reflects two distinct mechanisms: in the control samples, the absence of ionic species in ultrapure water and the lack of enzymatic activity—due to the absence of acetylcholine (ACh) substrate—result in low charge transfer and thus high impedance. Conversely, at low chlorpyrifos concentrations, enzymatic activity is inhibited, reducing the generation of ionic products near the sensor surface and leading to a drop in impedance. At higher concentrations, the impedance increases again, approaching control values, which may be explained by complex enzyme-surface interactions or compensatory charge redistribution at the electrode interface. These findings support the system’s ability to reliably distinguish between analyte concentrations and validate the overall electrochemical response pattern previously established.
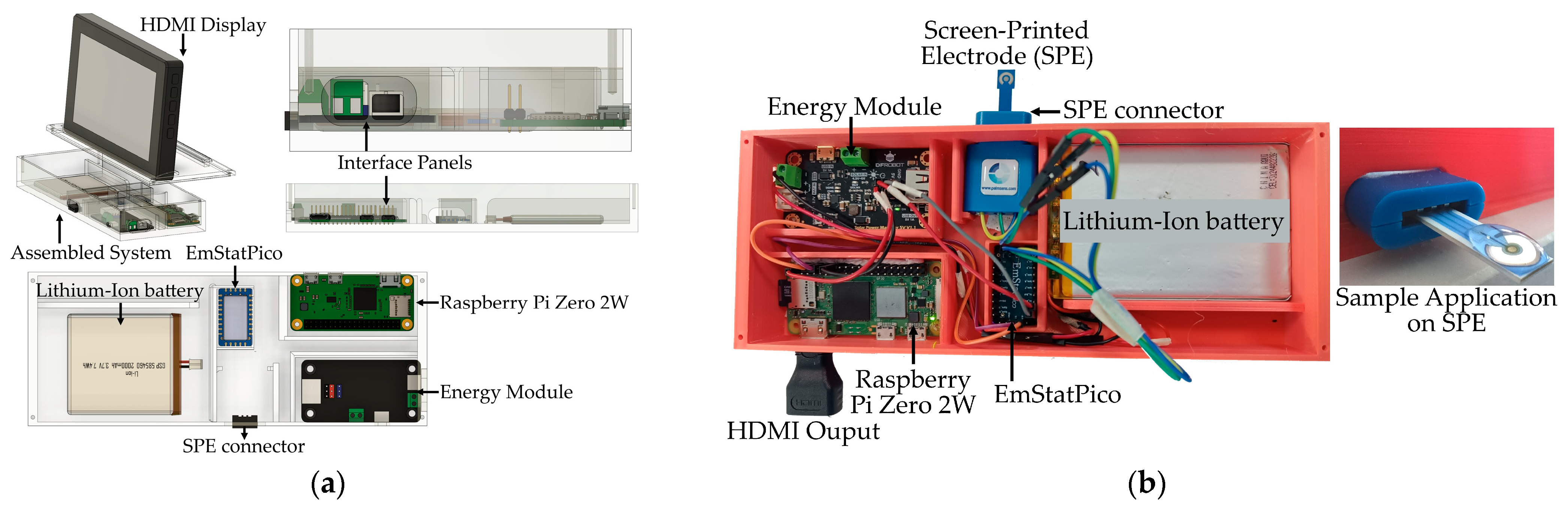
Figure 5a presents the 3D design of the implemented hardware, which integrates multiple modules to ensure portability and autonomous operation. The system includes an EmStatPico module for electrochemical measurements, a Raspberry Pi Zero 2W serving as a wireless data server, an HDMI display for data visualization in the absence of internet connectivity, a lithium-ion battery, and a solar charge manager module connected to a solar panel, which supplies power to all components.
Figure 5b shows the fully assembled system, highlighting its compact design and the connection to the electrochemical biosensor based on a screen-printed electrode (SPE). The biosensor is linked to the EmStatPico via a dedicated cable, and the liquid sample to be analyzed is applied directly onto the surface of the electrode.
4. Discussion
This work presents the development and evaluation of a portable system based on electrochemical impedance spectroscopy (EIS) for the detection of chlorpyrifos in aqueous samples. The platform integrates a commercial amperometric biosensor with immobilized acetylcholinesterase (AChE) as the biorecognition element, a PalmSens EmStatPico module for impedance measurement, and a Raspberry Pi Zero 2W for data transmission and local processing via custom Python software.
The system was evaluated using nine samples containing different concentrations of LorsbanTM 2.5% DP, a commercial formulation with 2.5% chlorpyrifos, prepared in distilled water. Electrochemical measurements were conducted using a frequency sweep from 20 Hz to 200 kHz, applying a sinusoidal signal of 15 mV over the open circuit potential (OCP). Impedance magnitude (|Z|) and phase angle (θ) were recorded at each frequency point.
The biosensor response exhibited the expected behavior of capacitive systems, with |Z| values decreasing at higher frequencies. Notably, samples with low chlorpyrifos concentrations (0.002 and 0.0058 g/mL) showed decreased impedance and decreased phase angle, while the highest concentration (0.0176 g/mL) exhibited values similar to the control group. This non-linear, biphasic response suggests that low pesticide levels suppress AChE activity, reducing ionic exchange and lowering the signal, whereas higher concentrations restore impedance values, potentially due to redistribution of charge at the electrode interface due to enzyme saturation effects. These patterns are consistent with prior studies employing EIS to detect enzymatic suppression by organophosphates [
13].
Quantitative analysis confirmed that the system distinguishes chlorpyrifos concentrations with statistical significance (F = 20.59,
p < 0.001), particularly between the control and the lower concentrations. In contrast, no statistically significant difference was observed between the control and the highest concentration tested. This behavior was further supported by post-hoc Tukey analysis and confidence intervals. The 100 Hz to 10 kHz range provided the best sensitivity and reproducibility, as supported by low standard deviations and consistent trends across replicates. The selection of this frequency window was guided by literature on AChE-based sensors, which identifies 100 Hz to 10 kHz as the optimal compromise between signal stability, sensitivity to interfacial effects, and minimization of noise artifacts [
11,
12,
13].
Conductivity values (
Table 3), however, did not follow a strictly linear trend with chlorpyrifos concentration. This observation suggests that the electrochemical signal is also modulated by secondary factors such as ionic mobility, localized variations in biosensor surface properties, and electrode–electrolyte interface dynamics.
Future studies should evaluate the system using real-world matrices (e.g., environmental or food samples) and compare its performance against standard analytical methods, such as high-performance liquid chromatography (HPLC) or gas chromatography–mass spectrometry (GC-MS), highlighting the need for further testing in complex sample environments to assess its robustness beyond controlled laboratory conditions.
5. Conclusions
This study demonstrated the feasibility and effectiveness of a portable electrochemical impedance spectroscopy (EIS) system for detecting chlorpyrifos in aqueous solutions. By integrating a commercial amperometric biosensor with immobilized acetylcholinesterase (AChE), a PalmSens EmStatPico module, and a Raspberry Pi Zero 2W, the system reliably detected concentration-dependent changes in impedance. The observed variations in impedance across concentration groups were statistically validated (F = 20.59, p < 0.001), confirming the analytical sensitivity of the platform for low chlorpyrifos concentrations.
Although electrical conductivity did not show a linear correlation with chlorpyrifos concentration, the consistent impedance trends across replicates and frequency ranges reaffirm the sensor’s reliability. These results confirm the potential of the developed system as a cost-effective, modular, and portable alternative to conventional laboratory-based methods for pesticide detection.
Future work should focus on validating the system with real environmental or food samples and comparing its performance with standard techniques such as high-performance liquid chromatography (HPLC) or gas chromatography–mass spectrometry (GC-MS). Subsequent work should focus on enhancing chemical selectivity, real-time data transmission, and robust calibration protocols to enable broader deployment in environmental monitoring contexts.