Abstract
Neurodegenerative diseases affect millions worldwide and present an urgent challenge due to the aging of the population. Drug delivery to the brain is limited by the blood–brain barrier (BBB), inspiring the development of nanotransporters like phytosomes. This study aimed to develop phosphatidylcholine (PC)-based phytosomes incorporating macroalgae extracts. Some of them were functionalized with PEG and ApoE to enhance BBB passage. The phytosomes were characterized by the encapsulation rate, size, polydispersity index (PDI), zeta potential, and stability, with BBB passage tested in an in vitro model (transwell hCMEC/D3 cell model). The phytosomes showed high stability and effective extract binding (74.9–80.3%) over four weeks. Although ApoE functionalization did not significantly improve BBB crossing, all nanotransporters successfully traversed the BBB in the model.
1. Introduction
Neurodegenerative diseases (NDs) occur when the nerve cells in the brain or peripheral nervous system gradually lose function and ultimately die. NDs affect millions of people worldwide and are becoming an imminent problem due to the rapid aging of the population [1,2,3]. Moreover, the scientific community and the public are increasingly interested in natural products with health-promoting characteristics [4].
Extracts obtained from various photosynthetic organisms exhibit bioactive properties, including neuroprotection. Macroalgae extracts stand out due to their rich contents of complex secondary metabolites such as phlorotannins, flavonoids, and phenolic acids, which are produced to ensure growth and survival in harsh conditions [5,6,7]. This work focused on the Fucales order, specifically on Bifurcaria bifurcata (BB), Fucus spiralis (FS), and Ascophyllum nodosum (AN). These brown macroalgae are found on the coasts of Europe and were selected due to their recognized health benefits, which include antioxidant, anti-inflammatory, antitumor, and neuroprotective properties [8,9,10,11]. However, the bioactive compounds responsible for these activities are large molecules (e.g., phlorotannins can be up to 650 kDa [12]), making their introduction into the human body difficult and leading to the necessity of increasing their bioavailability. For this purpose, several strategies are available such as amorphization, particle size reduction, or encapsulation into drug carrier systems such as nanoparticles, micelles, and phytosomes [13,14,15].
Phytosomes are artificial spherical vesicles formed by a concentric phospholipid bilayer that spontaneously organizes in an aqueous medium [16]. They simulate cellular membranes and are more stable that liposomes since, in phytosomes, the phytoconstituents are bound through H bonds to the polar heads, while in liposomes, the phytoconstituents are distributed in the polar cavity or in the apolar phospholipidic layers without any chemical interaction [17,18]. Phytosomes differ from others nanoparticles as they incorporate the bioactive substance into their structure via chemical bonds to the polar heads of phospholipids [18,19], forming an amphipathic complex that facilitates the passage through the blood–brain barrier (BBB) [20]. According to the literature, phytochemicals containing an active hydrogen atom (such as -COOH, -OH, -NH2, and -NH), like the bioactive polyphenols present in brown algae [21], can be incorporated into a phytosome structure [22]. The BBB is a selective, semipermeable membrane between the blood and the brain’s interstitial space that consists of endothelial cells, astroglia, pericytes, and microglia cells [23,24]. Paracellular obstruction through endothelial cell junctions serves as a shield, while other molecules are transported transcellularly from the blood to the brain via lipid-mediated free diffusion or carrier-mediated transport [25]. Due to their characteristics, such as biocompatibility, stability, and liposolubility, these nanocarriers are being studied for their potential pharmaceutical use [1]. Phytosomes typically utilize ligands like polyethylene glycol (PEG) to functionalize the nanoparticle surface, as PEG protects nanoparticles from binding to plasma proteins [26]. Furthermore, the immune system can recognize uncoated nanoparticles, leading to opsonization and phagocytosis [27]. Additionally, phytosomes can be modified with apolipoprotein E (ApoE). ApoE-functionalized nanoparticles can mimic lipoprotein particles, undergoing endocytosis at the BBB via low-density lipoprotein receptors [1,25]. Currently, treatment options for NDs are limited, mainly focusing on symptom relief [28]. The effective delivery of pharmacological agents to the brain is crucial, yet it is hindered by the presence of the BBB. This study aimed to incorporate bioactive extracts from macroalgae into phytosomes and characterize the resulting nanocarriers. Additionally, their ability to cross an endothelial cell layer simulating the BBB was evaluated.
2. Materials and Methods
2.1. Algae Extraction
The extracts of the three macroalgae studied (Bifurcaria bifurcata (BB), Fucus spiralis (FS), and Ascophyllum nodosum (AN), collected in the north of Spain) were obtained using microwave-assisted extraction (MAE). In brief, 0.6 g of lyophilized macroalgae was mixed with 20 mL of ethanol:water in closed vessels, and an irradiation power of 1400 W was applied. The mixture was kept under magnetic agitation, and the resulting extract was stored at −80 °C until further utilization [29,30].
2.2. Phytosome Production and Stability
The phytosomes were produced using a procedure described previously [31]. In brief, the phosphatidylcholine and the macroalga extract were combined 1:1 w/w, dissolved in ethanol, and kept at 60 °C for 1 h under agitation. After solvent evaporation, the complex was resuspended in CH3Cl and purified via double filtration through polytetrafluoroethylene (PTFE) filters (0.22 µm) and dried under a N2 flow. The entrapment rate of each extract was determined based on the absorbance at 280 nm. Phytosomes were further functionalized with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycerol)-2000) (DSPE-PEG(2000)) or DSPE-PEG(2000) plus ApoE. To assess the stability, the dried phytosomes were resuspended in CH3Cl, and their absorbance (250 nm) and spectra (200–500 nm) were read to check for differences. The process was repeated using the same phytosomes over 4 weeks [32].
2.3. Phytosome Characterization
Phytosomes were analyzed by UV–Vis spectrophotometry (Shimadzu 1900i, Tokyo, Japan), with the spectra obtained between 200 and 600 nm. Fourier-transform infrared spectroscopy (FTIR) (Nicolet 6700 Thermo Fisher Scientific, Waltham, MA, USA) was also conducted. Macroalgae extracts and phytosomes were finely pulverized and mixed with KBr (1:100) to form a pellet, while phosphatidylcholine was placed in a NaCl window. The spectra were then collected in the 400 to 4000 wavenumber range with a 4 cm−1 resolution. The size distribution and zeta potential were characterized by dynamic light scattering (DLS, Malvern Zetasizer ZS instrument, Worcestershire, UK). For that, samples were diluted in potassium phosphate buffer (0.1 M, pH 7.4), and measurements were performed using a folded capillary zeta cell [32,33].
2.4. hCMEC/D3 Cells Model of the Blood–Brain Barrier (BBB)
The ability of the phytosomes to cross the BBB was analyzed using a transwell hCMEC/D3 cell model, as previously described [34]. The phytosomes under study were dissolved in Hanks’ Balanced Salt Solution (HBSS) to a final concentration of 100 µg/mL and labeled with coumarin 6 (1% (w:w)), and 500 μL of the sample was introduced into the apical compartment (blood side), while 1.5 mL of HBSS was added to the basal compartment (brain side). Their passage through the simulated BBB was assessed using fluorescence. Emission was measured at 501 nm following excitation at 457 nm, after 3 and 24 h of incubation.
2.5. Statistical Analysis
The results are presented as mean ± SD of three replicates. Statistical comparisons were performed using ANOVA or t-test with GraphPad Prism software version 8.0.1.
3. Results and Discussion
The macroalgae extracts showed bioactive properties commonly related to the presence of phenolic compounds [21]. The presence of these compounds was estimated at 103, 85, and 64 mg phloroglucinol equivalents/g extract dw for AN, FS, and BB, respectively [29,30].
3.1. Entrapment Rate
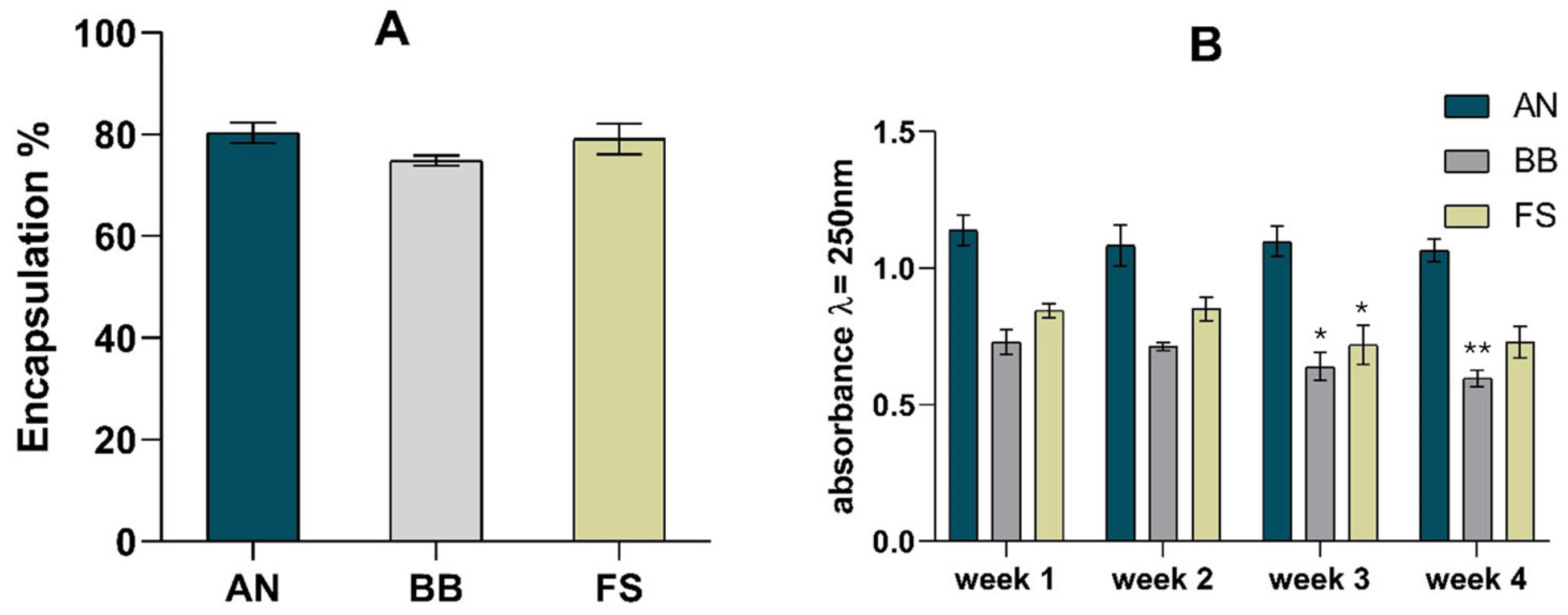
Five replicates of phytosomes were produced for each macroalgae extract. The concentration of the extracts bound to the phytosomes and the encapsulation rate were calculated using the absorbances measured at 280 nm (maximum absorbance of the extracts). Figure 1A shows the encapsulation rates obtained for the different extracts complexed with the phytosomes.
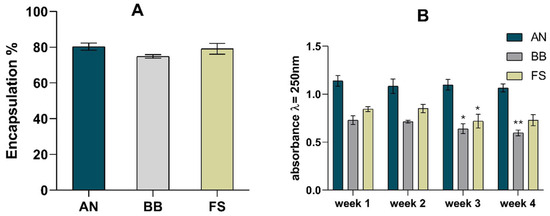
Figure 1.
(A) Phytosome encapsulation rate and (B) stability studies. For encapsulation rate, results were compared by ANOVA, followed by Tukey’s post hoc test, and no statistical differences were observed. For stability studies, results from weeks 2, 3, and 4 were compared with those of week 1. Statistically significant differences were determined by ANOVA, followed by Dunnett’s post hoc test, with * indicating p < 0.05 and ** indicating p < 0.01.
The encapsulation rates varied between 75% (BB) and 80% (AN). These results indicate a high encapsulation rate and reflect the extract bound to phosphatidylcholine. High entrapment efficiencies (94%) have been reported in a nanostructured lipid carrier [35] and for a lecithin-C-phycocyanin (1:1) phytosomal complex, where an entrapment efficiency of 88% was reported, highlighting the efficiency of this kind of process [36].
Likewise, the phosphatidylcholine-based phytosomes with Diospyros kaki L. acetonic extract also presented a high encapsulation efficiency (97%) [37]. A study of the stability of the phytosome complex was also performed, and the results are presented in Figure 1B. The absorbance measured at the maximum wavelength of the phytosomes (250 nm) remained stable for 4 weeks, with variations under 20%. Moreover, no changes appeared in the UV–Vis spectrum (200–500 nm), indicating that the complex was stable.
3.2. Phytosome Characterization
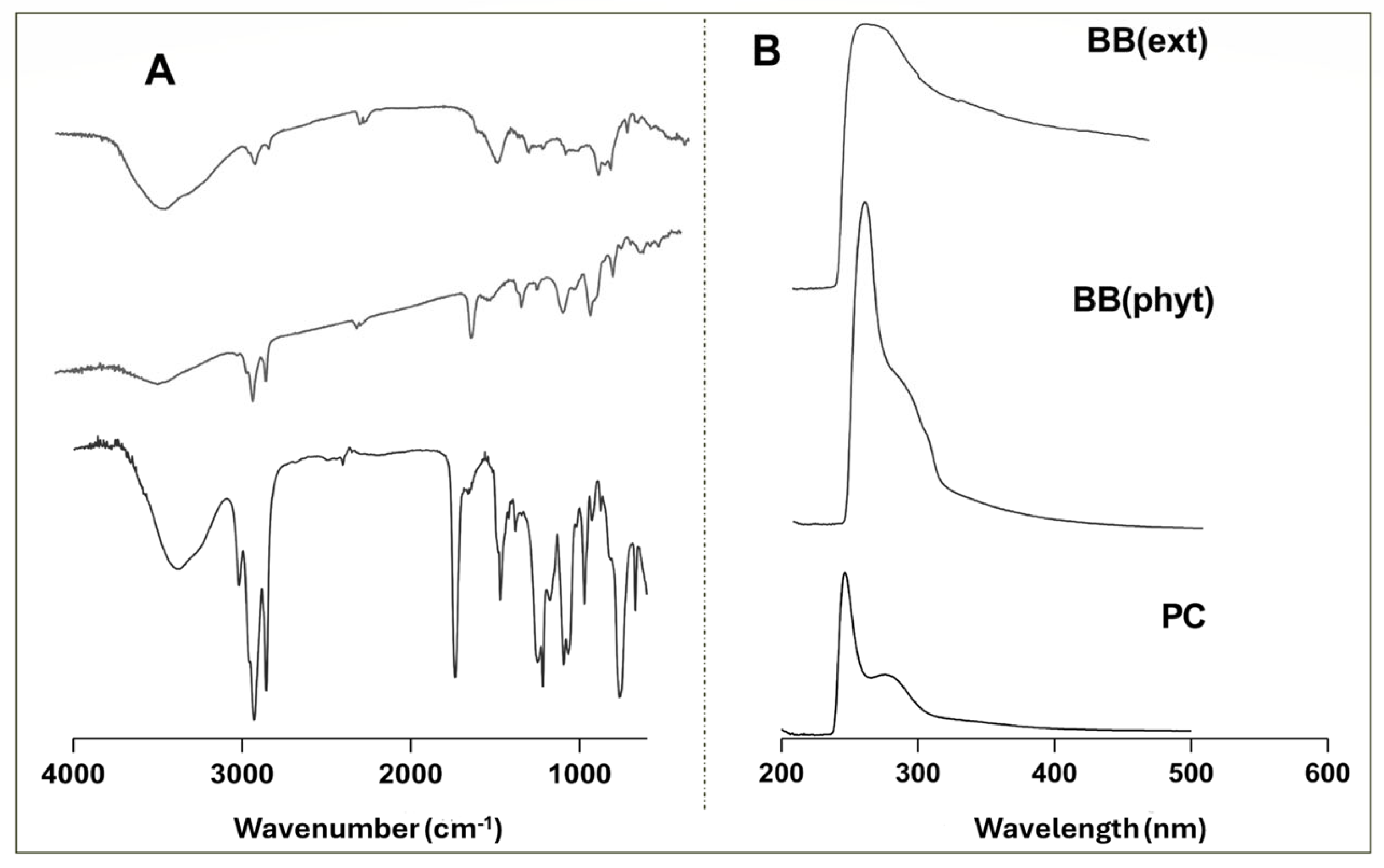
The phytosome complexes were characterized using spectroscopic techniques, including UV–Vis and FTIR, to evaluate the effectiveness of the encapsulation. As an example, the FTIR and UV–VIS spectra obtained for the BB extract (BB(ext)) and BB-based phytosome (BB(phyt)) are presented in Figure 2.
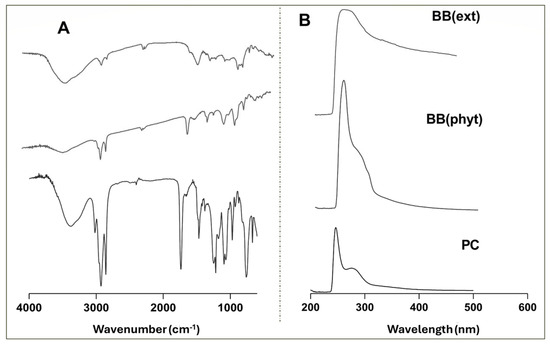
Figure 2.
Spectral profile of the BB extract, the phytosomes, and the phosphatidylcholine molecule: (A) FTIR; (B) UV–Vis.
In Figure 2A, the characteristic bands at 3400 cm−1 indicate the presence of the hydroxyl group (OH) in both the extract and the phospholipid complex. Groups such as C-H and C=O are common in both phytosomes and extracts as indicated by the bands at wavenumbers 3000 and 1700 cm−1, respectively. The P=O group presented a band at 1200 cm−1, and the C-O band appeared at 1100 cm−1. These functional groups were exclusive to the phytosome complex since these bonds did not previously appear in an intense form. On the other hand, the C-O-C group only appeared in the extracts (band near 1000 cm−1) [31,33,38]. Regarding the UV–Vis spectra (Figure 2B), the phytosome spectrum showed a clear narrowing of the band around 250 nm, confirming the inclusion of the extract in the phytosome structure. This behavior was similar in the three macroalgae tested and agrees with previous work describing the inclusion of Codium tomentosum algae into a phytosome nanostructure [31].
After the spectrophotometric characterization, the phytosomes were functionalized with DSPE-PEG(2000) and then with ApoE. Pegylation intends to protect phytosomes, while ApoE inclusion facilitates BBB transposition. The particle size was then evaluated, and the results are presented in Table 1.

Table 1.
Average size, polydispersity index, and zeta potential obtained by DLS for the different phytosomes.
Table 1 indicates the difference between the non-functionalized and functionalized phytosomes for the three extracts. The size of the phytosomes ranged from 118 (FS) to 168 (AN) nm, while the pegylated phytosomes varied between 254 (FS) and 366 (AN) nm. So, the size of the phytosomes increased due to functionalization, proving the creation of an additional layer around the nanotransporter.
Previous studies on the development of nanostructured lipid carriers with paclitaxel reported an average particle size of 121.44 ± 2.34 nm and a polydispersity index (PDI) of 0.114, which are in line with the presented results for the non-functionalized phytosomes [36]. Direito et al. [38] reported sizes of <300 nm and PDIs < 0.3 for phytosomal complexes with Diospyros kaki.
As for the PDI value, the functionalized phytosomes showed greater polydispersity, which was higher when only PEG was present compared to the combined presence of PEG and ApoE3, indicating that the non-functionalized phytosomes had a more uniform size distribution. Phytosomes developed with lecithin and C-phycocyanin as an active compound (with a lecithin to active compound ratio of 1:1) presented a polydispersity index of 0.379, which agrees with our results [35]. However, the authors observed that the PDI increased when larger amounts of phospholipids were used to produce the phytosomes (PDI = 0.474 for a 1:2 ratio and PDI = 0.682 for a 1:3 ratio). This could be related to the high lipid content, which enhanced the potential for agglomeration [35].
The zeta potential values indicate the stability of phytosomes in a medium. A value that is more positive than +30 mV or more negative than −30 mV typically signifies good stability against aggregation. While the values observed in this study did not exceed +30 mV or fall below −30 mV, it was found that adding an additional layer of PEG chains or PEG + ApoE to the phytosome surface reduced the zeta potential values. This result aligns with findings from previous studies [39].
3.3. In Vitro Effectiveness of Phytosomes’ Ability to Cross the BBB
To assess the nanocarriers’ capacity to cross the BBB, the phytosomes were tested before and after functionalization in a transwell hCMEC/D3 cell model. The results obtained are summarized in Table 2.

Table 2.
Percentage of non-functionalized and functionalized phytosomes permeating into the simulated BBB after 3 and 24 h of incubation.
For the phytosomes containing the AN extract, functionalization demonstrated a clear advantage over non-functionalized nanotransporters, with permeability rates of 5% compared to 0%. This suggests that incorporating ligands such as PEG and ApoE enhanced the permeability of these samples. In contrast, functionalization did not significantly impact the permeability of the BB and FS extract phytosomes, indicating that their transport across the BBB might involve mechanisms independent of ApoE transporters. At the 24 h time point, the permeability values were higher. However, the effect of functionalization during this period was unclear. These results are promising since a study demonstrated that Geophila repens phytosome systems, with their lower entrapment rates and higher particle size than the ones obtained herein, delivered phytocompounds intranasally, with their effects confirmed in an in vivo study [40].
4. Conclusions
This study incorporated natural macroalgae extracts into a lipid nanocarrier by developing phosphatidylcholine-based phytosomes. In general, functionalization led to higher particle sizes and PDI values. Also, the phytosomes produced were stable for at least 4 weeks. As for the capacity to cross the BBB, the functionalization of the phytosomes with ApoE did not prove to be a crucial step; nanotransporters passed through the hCMEC/D3 cell monolayer, regardless of their formulation. Future research should optimize and further explore the functionalization process.
Author Contributions
Conceptualization, A.S., M.C., C.G., C.R. and M.A.P.; methodology M.P., C.G., C.M. and A.I.O.; validation, A.S., M.C., C.G. and M.F.B.; formal analysis C.R., C.M., M.F.B. and M.A.P.; investigation M.P., A.S., A.I.O. and C.M.; resources, C.R. and M.A.P.; data curation A.I.O. and C.G.; writing—original draft preparation M.P., A.S. and M.C.; writing—review and editing A.S., C.G., C.R., M.A.P., M.F.B. and M.C.; supervision, C.R., M.F.B. and M.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from the PT national funds (FCT/MECI, Fundação para a Ciência e Tecnologia and Ministério da Educação, Ciência e Inovação) through the project UID/50006 -Laboratório Associado para a Química Verde—Tecnologias e Processos Limpos. and from Ibero-American Program on Science and Technology (CYTED—GENOPSYSEN, P222RT0117).
Data Availability Statement
Data is contained within this article.
Acknowledgments
Fatima Barroso (2020.03107.CEECIND) and Clara Grosso (CEECIND/03436/2020) thank FCT for their contract. Aurora Silva thanks the EU-FORA Fellowship Program (EUBA-EFSA-2023-ENREL-01) for the grant received.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grosso, C.; Silva, A.; Delerue-Matos, C.; Barroso, M.F. Single and Multitarget Systems for Drug Delivery and Detection: Up-to-Date Strategies for Brain Disorders. Pharmaceuticals 2023, 16, 1721. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.R.K.; Vijayakumar, K.K.; Murugesan, S.; Kunjiappan, S. Liposomes: An Emerging Carrier for Targeting Alzheimer’s and Parkinson’s Diseases. Heliyon 2022, 8, e09575. [Google Scholar] [CrossRef]
- Seo, M.W.; Park, T.E. Recent Advances with Liposomes as Drug Carriers for Treatment of Neurodegenerative Diseases. Biomed. Eng. Lett. 2021, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Al Jerdi, S.; MacDonald, R.; Triggle, C.R. Alzheimer’s Disease and Its Treatment–Yesterday, Today, and Tomorrow. Front. Pharmacol. 2024, 15, 1399121. [Google Scholar] [CrossRef]
- Guo, J.; Qi, M.; Chen, H.; Zhou, C.; Ruan, R.; Yan, X.; Cheng, P. Macroalgae-Derived Multifunctional Bioactive Substances: The Potential Applications for Food and Pharmaceuticals. Foods 2022, 11, 3455. [Google Scholar] [CrossRef]
- Rico, M.; González, A.G.; Santana-Casiano, M.; González-Dávila, M.; Pérez-Almeida, N.; de Tangil, M.S. Production of Primary and Secondary Metabolites Using Algae. In Prospects and Challenges in Algal Biotechnology; Springer Singapore: Singapore, 2017; pp. 311–326. [Google Scholar]
- Ambiga, S.; Pandian, R.S.; Lawrence, L.V.; Pandian, A.; Kumar, R.A.; Abdul, B.A.A. Marine Algal Secondary Metabolites Are a Potential Pharmaceutical Resource for Human Society Developments. In Marine Biochemistry; CRC Press: Boca Raton, FL, USA, 2022; pp. 339–362. [Google Scholar]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria Bifurcata in an in Vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A Concise Review of the Brown Macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Grina, F.; Ullah, Z.; Kaplaner, E.; Moujahid, A.; Eddoha, R.; Nasser, B.; Terzioğlu, P.; Yilmaz, M.A.; Ertaş, A.; Öztürk, M.; et al. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Fingerprints of Five Moroccan Seaweeds. S. Afr. J. Bot. 2020, 128, 152–160. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ramezanzade, L.; McClements, D.J. Recent Advances in Nanoencapsulation of Hydrophobic Marine Bioactives: Bioavailability, Safety, and Sensory Attributes of Nano-Fortified Functional Foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Leporatti, S.; Rinaldi, R. The New Frontiers in Neurodegenerative Diseases Treatment: Liposomal-Based Strategies. Front. Bioeng. Biotechnol. 2020, 8, 566767. [Google Scholar] [CrossRef]
- Bhagyashree, H.A.P. Phytosome as a Novel Biomedicine: A Microencapsulated Drug Delivery System. J. Bioanal. Biomed. 2015, 7, 6–12. [Google Scholar] [CrossRef]
- Mehmood, H. Phytosome as a Novel Carrier for Delivery of Phytochemicals: A Comprehensive Review. Middle East J. Appl. Sci. Technol. 2023, 6, 21–51. [Google Scholar] [CrossRef]
- Chaudhary, K.; Rajora, A. Phytosomes: A Critical Tool for Delivery of Herbal Drugs for Cancer. Phytochem. Rev. 2024, 24, 165–195. [Google Scholar] [CrossRef]
- Kanojiya, D.; Parmar, G.; Chauhan, B.; Gondalia, S.; Rakholiya, M. Phytosomes: A Contemporary Method for Delivering Novel Herbal Drugs. J. Nat. Remedies 2024, 24, 239–253. [Google Scholar] [CrossRef]
- Ozay, C.; Karpuz, M. Phytocompounds and Lipid-Based Drug Delivery System for Neurodegenerative Diseases. Asian Pac. J. Trop. Biomed. 2024, 14, 417–426. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Potential Pharmacological Applications of Polyphenolic Derivatives from Marine Brown Algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Bian, Z.; Chen, X.; Lu, A.; Yang, Z. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs Across the Blood-Brain Barrier. Focus 2022, 20, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Nardo, L.; Gregori, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, C. Functionalized Liposomes and Phytosomes Loading Annona Muricata L. Aqueous Extract: Potential Nanoshuttles for Brain-Delivery of Phenolic Compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Mehta, P.; Shende, P. Evasion of Opsonization of Macromolecules Using Novel Surface-Modification and Biological-Camouflage-Mediated Techniques for next-Generation Drug Delivery. Cell Biochem. Funct. 2023, 41, 1031–1043. [Google Scholar] [CrossRef]
- Michielin Lopes, C.; de Camargo, R.W.; Bitencourt, R.M. Doenças Neurodegenerativas e Canabinoides: Revisão Narrativa. Rev. Neurociências 2023, 31, 1–27. [Google Scholar] [CrossRef]
- Cassani, L.; Silva, A.; Carpena, M.; Pellegrini, M.C.; García-Pérez, P.; Grosso, C.; Barroso, M.F.; Simal-Gandara, J.; Gómez-Zavaglia, A.; Prieto, M.A. Phytochemical Compounds with Promising Biological Activities from Ascophyllum Nodosum Extracts Using Microwave-Assisted Extraction. Food Chem. 2024, 438, 138037. [Google Scholar] [CrossRef]
- Silva, A.; Carpena, M.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Delerue-Matos, C.; Simal-Gandara, J.; Barroso, M.F.; Prieto, M.A. Optimization and Bioactive Evaluation of Bifurcaria Bifurcata Antioxidant-Rich Extracts for Functional Food and Pharmaceutical Applications. Antioxidants 2024, 13, 1189. [Google Scholar] [CrossRef]
- Costa, M.; Soares, C.; Silva, A.; Grosso, C.; Delerue-Matos, C. Characterization of Codium Tomentosum Phytosomes and Their Neuroprotective Potential. Biol. Life Sci. Forum 2022, 18, 35. [Google Scholar]
- Costa, M.; Soares, C.; Silva, A.; Barroso, M.F.; Simões, P.; Ferreira, M.; Gameiro, P.; Grosso, C.; Delerue-matos, C. Optimization of Nanoencapsulation of Codium Tomentosum Extract and Its Potential Application in Yogurt Fortification. Mar. Drugs 2025, 24, 147. [Google Scholar] [CrossRef]
- Kumar, S.; Baldi, A.; Sharma, D.K. Characterization and In Vitro Investigation of Antiscabietic Effect of Phytosomes Assimilating Quercetin and Naringenin Rich Fraction of Pistacia Integerrima Galls Extract against Sarcoptes Scabiei. J. Drug Deliv. Sci. Technol. 2022, 67, 102851. [Google Scholar] [CrossRef]
- Cayero-Otero, M.D.; Gomes, M.J.; Martins, C.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Sarmento, B.; Martín-Banderas, L. In Vivo Biodistribution of Venlafaxine-PLGA Nanoparticles for Brain Delivery: Plain vs. Functionalized Nanoparticles. Expert Opin. Drug Deliv. 2019, 16, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.N.; Negm El-Dein, A.; Abd Al-Daim, S.; Allayeh, A.; Awad, H.; Flefil, N.S. Characterization of C-Phycocyanin Antioxidant, Anti-Inflammatory, Anti-Tumour, and Anti-HCoV-229E Activities and Encapsulation for Implementation in an Innovative Functional Yogurt. Heliyon 2024, 10, e31642. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Singla, M.; Smriti; Kapoor, R.; Kumar, D.; Gupta, S.; Gupta, G.; Bhattacharya, T. Paclitaxel Loaded Capmul MCM and Tristearin Based Nanostructured Lipid Carriers (NLCs) for Glioblastoma Treatment: Screening of Formulation Components by Quality by Design (QbD) Approach. Discov. Nano 2024, 19, 175. [Google Scholar] [CrossRef]
- Direito, R.; Reis, C.; Roque, L.; Gonçalves, M.; Sanches-Silva, A.; Gaspar, M.M.; Pinto, R.; Rocha, J.; Sepodes, B.; Rosário Bronze, M.; et al. Phytosomes with Persimmon (Diospyros kaki L.) Extract: Preparation and Preliminary Demonstration of In Vivo Tolerability. Pharmaceutics 2019, 11, 296. [Google Scholar] [CrossRef]
- Semalty, A.; Tanwar, Y.S.; Singh, D.; Rawat, M.S.M. Phosphatidylcholine Complex in Improving Oral Drug Delivery of Epicatechin: Preparation and Characterization. Drug Discov. Dev. 2014, 1, 46–55. [Google Scholar]
- Rabanel, J.-M.; Hildgen, P.; Banquy, X. Assessment of PEG on Polymeric Particles Surface, a Key Step in Drug Carrier Translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef]
- Rajamma, S.S.; Krishnaswami, V.; Prabu, S.L.; Kandasamy, R. Geophila Repens Phytosome-Loaded Intranasal Gel with Improved Nasal Permeation for the Effective Treatment of Alzheimer’s Disease. J. Drug Deliv. Sci. Technol. 2022, 69, 103087. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).