Determination of Escherichia coli in Raw and Pasteurized Milk Using a Piezoelectric Gas Sensor Array †
Abstract
1. Introduction
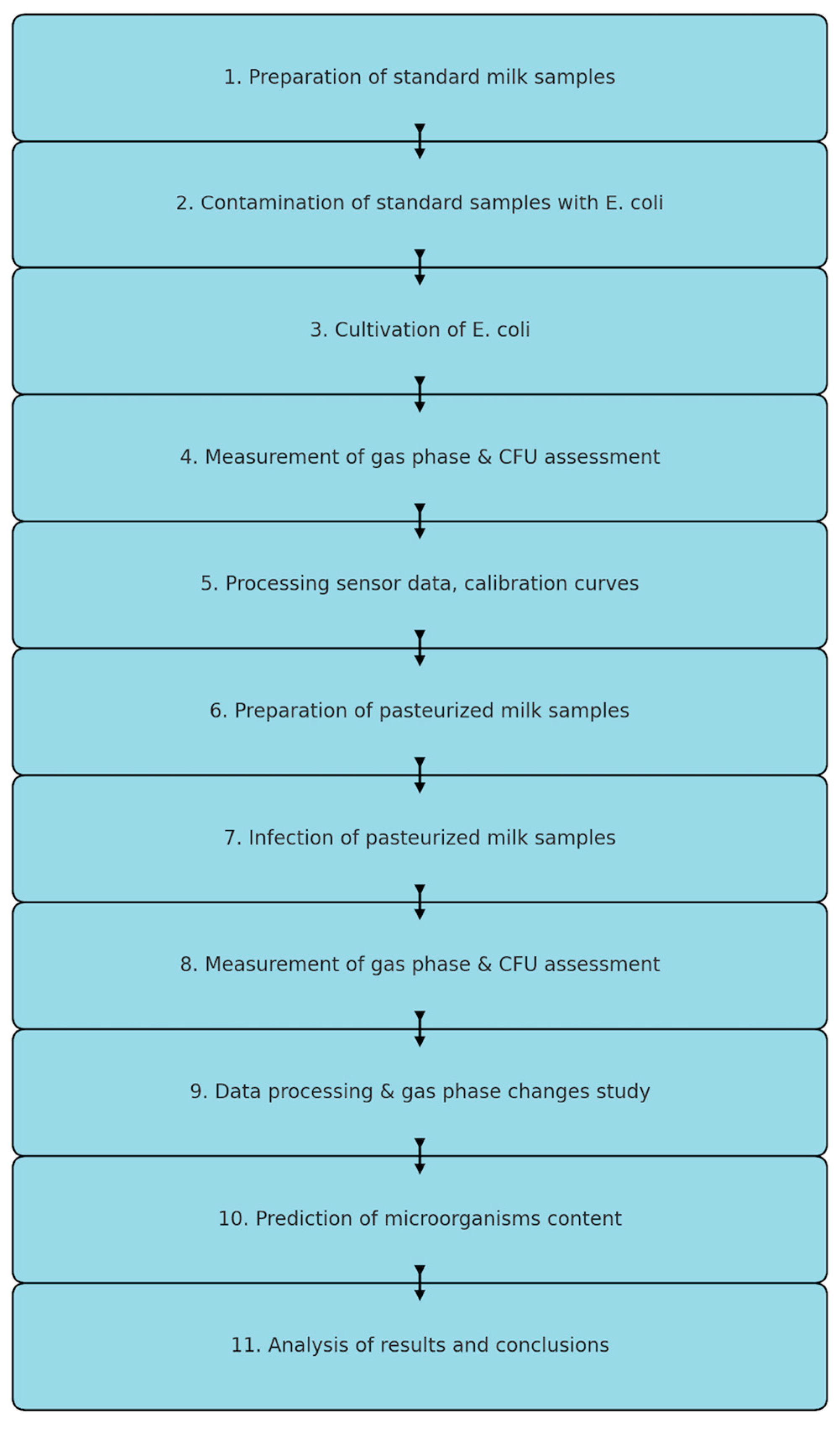
2. Materials and Methods
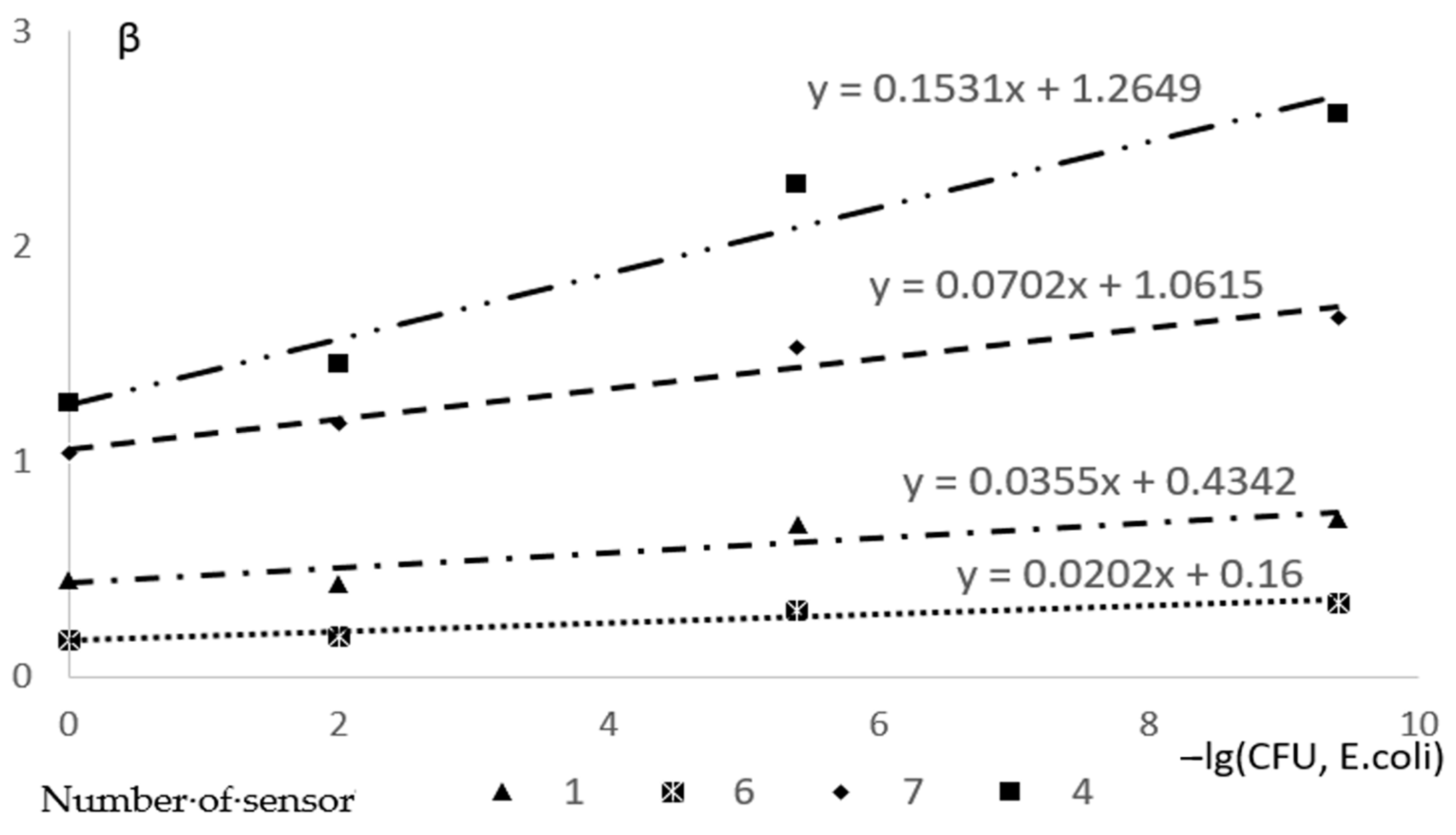
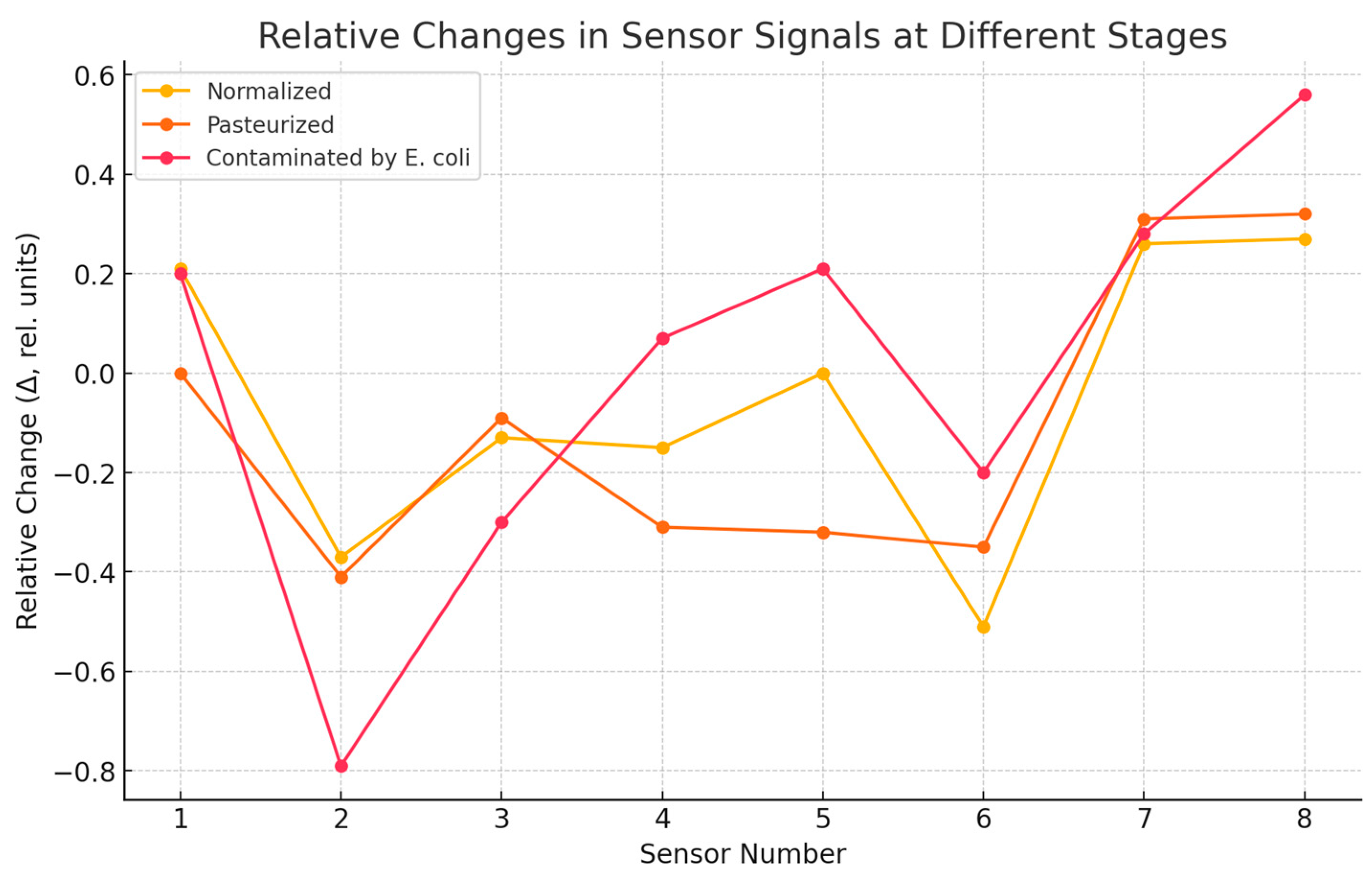
3. Results and Discussion
- Use experimental design methods to assess the content of various microorganisms in milk.
- Conduct repeated studies using raw milk with a wider variation in physicochemical parameters and in different seasons to account for the variation in the change in the native gas composition of milk depending on these factors.
- Conduct additional studies to increase the accuracy of the analysis and take into account all possible factors that can affect the experiment.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OJSC | Open joint stock company |
| DNA | Deoxyribonucleic acid |
| PJSC | Public joint stock company |
| PCR | Polymerase chain reaction |
| CFU | Colony-forming unit |
References
- Vakili, V.; Vakili, K.; Zamiri Bidari, M.; Azarshab, A.; Vakilzadeh, M.M.; Kazempour, K. Effect of Social Beliefs on Consumption of Dairy Products and Its Predicting Factors Based on the Transtheoretical Model: A Population-Based Study. J. Environ. Public Health 2023, 2023, 5490068. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Shrivastava, S.L. Recent Trends in Milk Processing-A Short Review. Approaches Poult. Dairy Vet. Sci. 2017, 2, 108–110. [Google Scholar] [CrossRef]
- De Corato, U. Soil Microbiota Manipulation and Its Role in Suppressing Soil-Borne Plant Pathogens in Organic Farming Systems under the Light of Microbiome-Assisted Strategies. Chem. Biol. Technol. Agric. 2020, 7, 17. [Google Scholar] [CrossRef]
- Sarmah, P.; Dan, M.M.; Adapa, D.; Tk, S. A Review on Common Pathogenic Microorganisms and Their Impact on Human Health. Electron. J. Biol. 2018, 14, 50–58. [Google Scholar]
- Paraffin, A.S.; Zindove, T.J.; Chimonyo, M. Effect of Structural Condition of Milk Processing Facilities and Food Safety Systems on Escherichia coli and Coliforms Presence in Cultured Buttermilk. J. Food Qual. 2019, 2019, 7365983. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, J.; Guo, Y.; Ma, X.; Sun, C.; Cai, M.; Chi, Y.; Xu, K. Application of ATP-Based Bioluminescence Technology in Bacterial Detection: A Review. Analyst 2023, 148, 3452–3459. [Google Scholar] [CrossRef]
- Patil-Joshi, A.; Rangaswamy, B.E.; Apte-Deshpande, A. Paper-Based PCR Method Development, Validation and Application for Microbial Detection. J. Genet. Eng. Biotechnol. 2021, 19, 37. [Google Scholar] [CrossRef]
- He, X.; Zou, B.; Qi, X.; Chen, S.; Lu, Y.; Huang, Q.; Zhou, G. Methods of Isothermal Nucleic Acid Amplification-Based Microfluidic Chips for Pathogen Microorganism Detection. Hereditas 2019, 41, 611–624. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, D. Isothermal Nucleic Acid Amplification and Its Uses in Modern Diagnostic Technologies. 3 Biotech 2023, 13, 200. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Han, X.; Feng, Q.; Wang, P. Combination of DNA Walker and Pb2+-Specific DNAzyme-Based Signal Amplification with a Signal-off Electrochemical DNA Sensor for Staphylococcus Aureus Detection. Anal. Chim. Acta 2022, 1222, 340179. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Suthindhiran, K. Development of Magnetosomes-Based Biosensor for the Detection of Listeria Monocytogenes from Food Sample. IET Nanobiotechnol. 2020, 14, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, P.; Kasaei, B.; Aghili, S.; Monirvaghefi, A.; Hosseini, A.; Amoozegar, H.; Mirfendereski, G.; Razzaghi, H. Application of Nanobiosensors in Detection of Pathogenic Bacteria: An Update. Res. Biotechnol. Environ. Sci. 2023, 2, 65–74. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Huang, Q.; Tong, T.; Zhou, Y.; Li, Z.; Bai, Q.; Liang, H.; Chen, L. Recent Advances on Aptamer-Based Biosensors for Detection of Pathogenic Bacteria. World J. Microbiol. Biotechnol. 2021, 37, 45. [Google Scholar] [CrossRef] [PubMed]
- Ropero-Vega, J.L.; Albiares-Sánchez, L.J.; Leon-Sanchez, W.R.; Valdivieso-Quintero, W.; Florez-Castillo, J.M. Detection of Pathogenic E. coli by Electrochemical Biosensors Based on Aptamers Designed by Bioinformatic Tools. Chem. Eng. Trans. 2022, 93, 283–288. [Google Scholar] [CrossRef]
- Spagnolo, S.; De La Franier, B.; Davoudian, K.; Hianik, T.; Thompson, M. Detection of E. Coli Bacteria in Milk by an Acoustic Wave Aptasensor with an Anti-Fouling Coating. Sensors 2022, 22, 1853. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, P.; Yan, Z.; Zhou, Y.; Du, Y.; Qu, C.; Song, Y.; Zhou, D.; Qu, S.; et al. Cell-Based Fluorescent Microsphere Incorporated with Carbon Dots as a Sensitive Immunosensor for the Rapid Detection of Escherichia coli O157 in Milk. Biosens. Bioelectron. 2021, 179, 113057. [Google Scholar] [CrossRef]
- Malvano, F.; Pilloton, R.; Albanese, D. A Novel Impedimetric Biosensor Based on the Antimicrobial Activity of the Peptide Nisin for the Detection of Salmonella spp. Food Chem. 2020, 325, 126868. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. Trends in Artificial Aroma Sensing by Means of Electronic Nose Technologies to Advance Dairy Production–A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 234–248. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, L. Application of E-Nose Technology Combined with Artificial Neural Network to Predict Total Bacterial Count in Milk. J. Dairy Sci. 2021, 104, 10558–10565. [Google Scholar] [CrossRef]
- Carrillo-Gómez, J.K.; Durán Acevedo, C.M.; García-Rico, R.O. Detection of the Bacteria Concentration Level in Pasteurized Milk by Using Two Different Artificial Multisensory Methods. Sens. Bio-Sens. Res. 2021, 33, 100428. [Google Scholar] [CrossRef]
- Shuba, A.A.; Bogdanova, E.V.; Anokhina, E.P.; Umarkhanov, R.U. Current trends in the determination of microbiological indicators of dairy products. J. Food Sci. Technol. 2025, 62, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Kuchmenko, T. Chemical Sensors Based on Piezoelectric Quartz Microbalances. In Chemical Sensors, 1st ed.; Vlasov, Y.G., Ed.; Nauka: Moscow, Russia, 2011; Volume 14, pp. 120–195. ISBN 978-5-02-037511-6. [Google Scholar]
- Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials 2021, 14, 4263. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW Sensors for Chemical Vapors and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef]
- Shuba, A.; Anokhina, E.; Umarkhanov, R.; Bogdanova, E. Development of a Chemical Sensor Based on Deep Eutectic Solvents and Its Application for Milk Analysis. Eng. Proc. 2023, 48, 34. [Google Scholar] [CrossRef]
- Reis, M.G.; Harris, P.; Berry, C.; Nguyen, H.; Maclean, P.; Weeks, M. Tracking Changes in Volatile Components and Lipids after Homogenisation and Thermal Processing of Milk. Int. Dairy J. 2020, 103, 104624. [Google Scholar] [CrossRef]
| Type of Sensor | Limit of VOC Detection | Advantages | Disadvantages |
|---|---|---|---|
| Chemoresistive | 5–500 ppm | High sensitivity, low operating temperature, and a thermal stable structure, simplicity, low cost, small size, and ability to be integrated into electronic devices | High sensitivity to water vapor, high possibility of sensor poisoning, low selectivity |
| Optical | 1 ppm–1000 ppb | Commercial availability, simplicity of sensor formation | The complexity of creating devices, fluorescent dyes have a short operating time |
| Metal oxide | 1–1000 ppm | Low power consumption, the possibility of long battery life, long life of the sensor material, ability to work in explosive environments | Low selectivity, poor sensitivity to organic molecules and relatively low stability caused by recrystallization and surface poisoning processes |
| Piezoelectric quartz microbalance | 10 ppm–10 ppb | Linear calibration curve over a wide concentration range, fast response and recovery time, high sensitivity | Fragile sensing element, possibility of electrode corrosion |
| Surface acoustic waves (SAW) | 1 ppm–1 ppb | High sensitivity, excellent response time, small size, low cost, ability to work in wired and wireless mode | Membrane aging |
| Name of the Indicator | Sample | ||||||
|---|---|---|---|---|---|---|---|
| Reference No 1 | Raw Cow’s Milk | Skim Milk | Normalized Mixture | Pasteurized Mixture | Drinking Milk (Experimental) | Drinking Milk (Control) | |
| Milk in dry matter, % | 12.3–12.5 | 11.36 ± 0.30 | 9.71 ± 0.17 | 11.50 ± 0.38 | 11.58 ± 0.33 | 11.53 ± 0.41 | |
| Fat in dry matter, % | 4.0–4.1 | 3.05 ± 0.05 | 0.05 ± 0.05 | 2.5 ± 0.05 | |||
| Total protein in dry matter, % | 3.0–3.1 | 3.46 ± 0.1 | |||||
| Lactose in dry matter, % | 4.65–4.70 | 4.41 ± 0.36 | Not determined | 3.47 ± 0.15 | 3.10 ± 0.29 | 3.35 ± 0.20 | |
| Titratable acidity, °T | 17 ± 0.5 | 18 ± 0.5 | 19 ± 0.5 | 17 ± 0.5 | 18 ± 0.5 | 18 ± 0.5 | 18 ± 0.5 |
| Density, kg/m3 | 1025 ± 0.5 | 1031 ± 0.5 | 1030 ± 0.5 | 1026 ± 0.5 | 1026 ± 0.5 | 1026 ± 0.5 | 1026 ± 0.5 |
| QMAFAnM, CFU/mL | <103 | 5.8×106 | Not determined | <103 | Not determined | ||
| Coliform bacteria, CFU/mL | 0 | Not determined | 0 | 102 | 0 | ||
| Sensor Number for Calibration | Milk Samples | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | −0.67 | 0.41 | −1.40 | 3.66 | 0.77 | 4.74 | 2.21 |
| 4 | 4.47 | 3.04 | 1.79 | 5.56 | 3.88 | 4.89 | 4.47 |
| 6 | 0.52 | 1.79 | −2.02 | 3.06 | 1.15 | 3.06 | 3.06 |
| 7 | −1.29 | 1.45 | −3.48 | 4.01 | 1.81 | 2.36 | 0.90 |
| CFU(E. coli) in cm3 | <10 | 102 | <10 | 0 | 102 | 0 | 103 |
| Notes | Presence of other pathogenic microorganisms | High level of QMAFAnM (>106) in raw milk | Artificial contaminated | Presence of mold (>600) in raw milk | Artificial contaminated | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuba, A.; Umarkhanov, R.; Bogdanova, E.; Anokhina, E.; Burakova, I. Determination of Escherichia coli in Raw and Pasteurized Milk Using a Piezoelectric Gas Sensor Array. Eng. Proc. 2025, 87, 31. https://doi.org/10.3390/engproc2025087031
Shuba A, Umarkhanov R, Bogdanova E, Anokhina E, Burakova I. Determination of Escherichia coli in Raw and Pasteurized Milk Using a Piezoelectric Gas Sensor Array. Engineering Proceedings. 2025; 87(1):31. https://doi.org/10.3390/engproc2025087031
Chicago/Turabian StyleShuba, Anastasiia, Ruslan Umarkhanov, Ekaterina Bogdanova, Ekaterina Anokhina, and Inna Burakova. 2025. "Determination of Escherichia coli in Raw and Pasteurized Milk Using a Piezoelectric Gas Sensor Array" Engineering Proceedings 87, no. 1: 31. https://doi.org/10.3390/engproc2025087031
APA StyleShuba, A., Umarkhanov, R., Bogdanova, E., Anokhina, E., & Burakova, I. (2025). Determination of Escherichia coli in Raw and Pasteurized Milk Using a Piezoelectric Gas Sensor Array. Engineering Proceedings, 87(1), 31. https://doi.org/10.3390/engproc2025087031







