1. Introduction
Butadiene 1,3 is an essential feedstock in the petrochemical industry, primarily utilized in the synthesis of synthetic rubber, latex, and various polymeric materials [
1]. It is predominantly obtained from the cracking of hydrocarbons in naphtha or gas oil-based steam-cracking units [
2]. The separation of butadiene from pyrolysis products is challenging due to its close boiling point with other C4 hydrocarbons such as butenes and butanes [
3]. Extractive distillation, often employing selective solvents like N-methyl-2-pyrrolidone (NMP) or acetonitrile, is widely used to achieve the desired purity and recovery rates [
4].
These advancements provide valuable context for further improving butadiene separation efficiency and sustainability beyond conventional extractive distillation approaches.
Process optimization is crucial for minimizing energy consumption while ensuring high product quality. Advanced modeling and simulation tools, such as Aspen Plus, have proven effective in optimizing separation processes by allowing precise evaluation of operational parameters [
5]. A study has explored the impact of variables such as solvent-to-feed ratio, reflux ratio, and column configurations on butadiene separation [
6]. By integrating simulation with experimental validation, efficiency improvements of more than 20% in energy savings have been reported [
7].
In recent years, significant research efforts have been dedicated to exploring alternative solvents and advanced separation methods to enhance butadiene recovery. Novel solvents such as ionic liquids and deep eutectic solvents have been investigated for their high selectivity and reduced environmental impact compared to conventional organic solvents [
8]. Furthermore, membrane-based separation technologies have emerged as promising alternatives, offering the potential for lower energy consumption and operational flexibility [
9]. Hybrid separation processes, combining extractive distillation with pervaporation or adsorptive separation, are also being explored to overcome the limitations of traditional distillation [
10].
In this study, an Aspen Plus-based model was developed to investigate the separation of butadiene 1,3 from pyrolysis products. The analysis focuses on key operational parameters including solvent-to-feed ratio, reflux ratio, theoretical stages, and energy integration strategies. Experimental data from industrial-scale units were utilized to validate the model, ensuring applicability to real-world scenarios [
11]. The goal of this research is to provide insights into optimizing butadiene recovery, reducing energy consumption, and improving the overall economic viability of the process. The findings presented in this study contribute to the broader efforts in enhancing efficiency and sustainability in petrochemical separations.
2. Background
Butadiene is typically produced through steam cracking of hydrocarbons such as naphtha and gas oil, yielding a mixture of C4 hydrocarbons that require further separation [
2]. Extractive distillation is the preferred method for butadiene separation, as conventional distillation is ineffective due to the close boiling points of C4 components [
12].
The production and separation of butadiene 1,3 from pyrolysis products involve several key chemical reactions (Equations (1)–(7)), primarily occurring during the steam cracking of hydrocarbons and subsequent separation processes.
2.1. Steam Cracking
Steam cracking is a thermal decomposition process where hydrocarbons are broken down into smaller molecules at high temperatures (800–900 °C) in the presence of steam. The general reaction for the cracking of a hydrocarbon (e.g., naphtha) can be represented as follows [
13]:
For example, the cracking of naphtha (a mixture of C
5–C
12 hydrocarbons) can produce butadiene (C
4H
6) along with other olefins like ethylene (C
2H
4) and propylene (C
3H
6). A simplified reaction for the formation of butadiene from a C
4 hydrocarbon (e.g., butane) is the following:
The C4 stream from steam cracking contains a mixture of butadiene, butenes (1-butene, 2-butene, and isobutene), and butanes. The separation of butadiene from this mixture is challenging due to the similar boiling points of these components.
2.2. Conventional Distillation Limitations
Traditional conventional distillation methods are generally ineffective for separating C4 hydrocarbons, including butadiene, because their boiling points are too close to each other. For instance:
- –
1,3-Butadiene boils at −4.4 °C
- –
1-Butene boils at −6.3 °C
- –
n-Butane boils at −0.5 °C
These similarities make achieving high-purity separation by standard distillation extremely energy-intensive and inefficient. As a result, alternative separation methods are necessary for industrial viability [
7].
2.3. Extractive Distillation
Extractive distillation is employed, using a solvent that selectively interacts with butadiene to increase its relative volatility. The process can be summarized as follows:
The C4 mixture is fed into a distillation column along with a solvent (e.g., DMF, ACN, or NMP). The solvent forms a complex with butadiene, allowing it to be separated from the other C4 components. The reaction between butadiene and the solvent can be represented as follows [
14]:
Stripping/Recovery: The butadiene-rich solvent is sent to a stripper or recovery column, where heat is applied to break the butadiene–solvent complex. The stripper operates typically between 30 and 150 °C depending on the solvent used [
4]. The butadiene is released as a vapor, and the solvent is regenerated for reuse. The reaction can be represented as follows:
The purified butadiene is then used as a monomer in the production of synthetic rubbers and polymers. For example, butadiene reacts with styrene to produce styrene-butadiene rubber (SBR):
Similarly, butadiene polymerizes to form polybutadiene rubber (PBR):
Butadiene is also a key component in the production of acrylonitrile-butadiene-styrene (ABS) resins, which are widely used in automotive and consumer goods industries. The copolymerization reaction for ABS can be represented as follows:
In summary, the production and separation of butadiene involve a series of chemical reactions, including hydrocarbon cracking, extractive distillation, and polymerization. The production of ABS resins involves a generalized copolymerization process between acrylonitrile, butadiene, and styrene monomers.
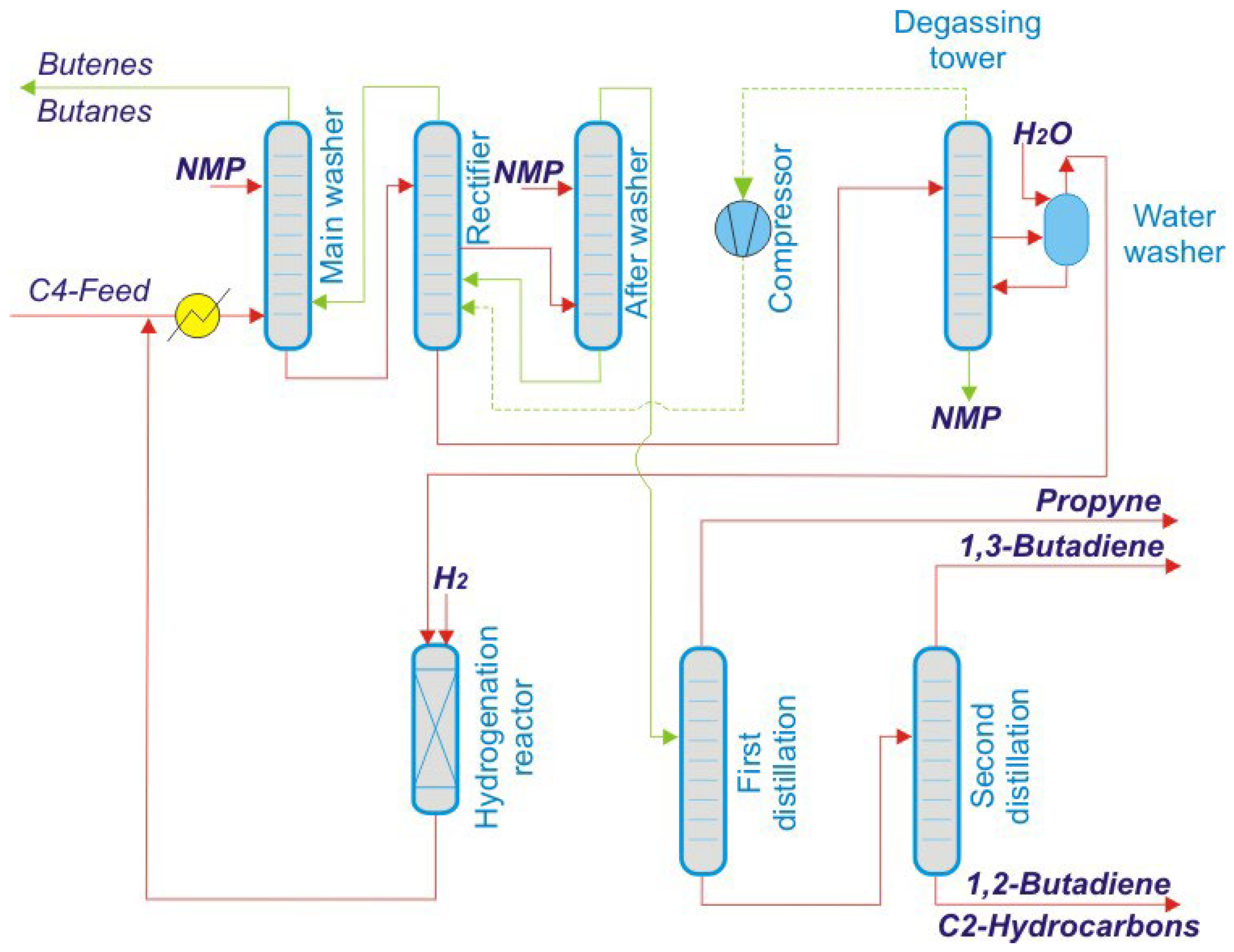
These processes are critical for meeting the demand for synthetic rubbers and polymers in various industries. The overall production and separation process of butadiene is illustrated in
Figure 1. Ongoing advancements in separation technologies, such as the development of more selective solvents or membrane-based processes, aim to improve the efficiency and sustainability of butadiene production [
14].
2.4. Comparative Analysis of Solvents: DMF, ACN, and NMP
In extractive distillation processes for the separation of butadiene from C
4 hydrocarbons, the choice of solvent plays a critical role in determining the efficiency, selectivity, and sustainability of the operation. Commonly used solvents include N-methyl-2-pyrrolidone (NMP), dimethylformamide (DMF), and acetonitrile (ACN). Each of these solvents offers distinct advantages and disadvantages (
Table 1):
Among these, NMP is often preferred in industrial applications due to its superior selectivity and operational robustness despite its higher cost [
12]. DMF offers moderate selectivity but raises health and environmental concerns due to its toxicity. ACN, although less selective, offers operational advantages in terms of energy efficiency and process control owing to its low boiling point and viscosity.
Thus, solvent selection must balance separation performance, operational cost, and safety/environmental considerations for optimized butadiene recovery.
3. Methodology and Simulation
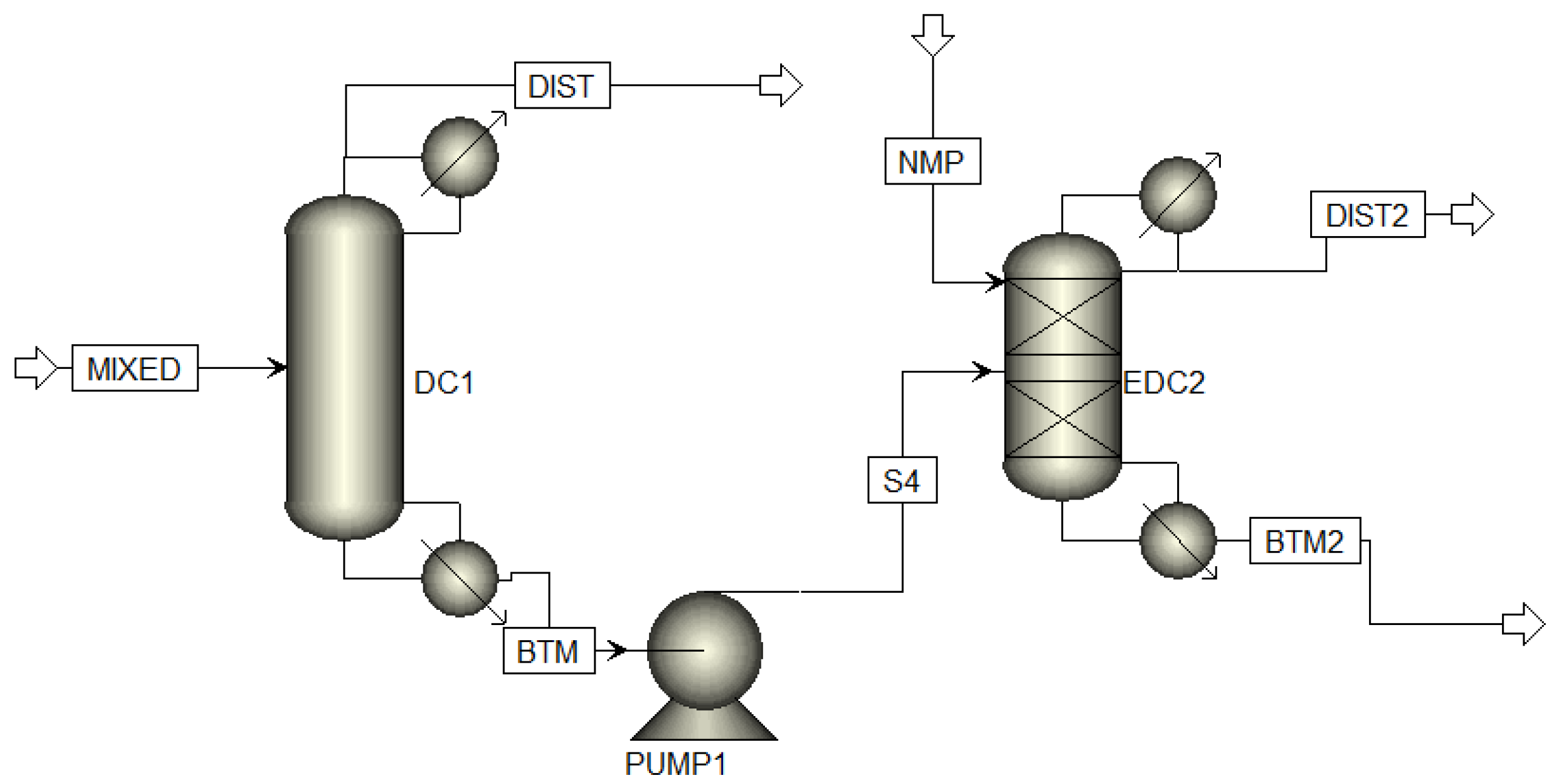
The separation of butadiene 1,3 was modeled using Aspen Plus, incorporating an extractive distillation unit with a selective solvent to enhance the separation from C4 hydrocarbons (
Figure 2). The simulation used a rigorous thermodynamic model, specifically the Non-Random Two-Liquid (NRTL) model, to accurately predict phase behavior and separation efficiency. The simulation considered process optimization parameters such as solvent-to-feed ratio, reflux ratio, number of theoretical stages, and overall heat integration to improve energy efficiency. The NRTL model is based on the concept of local composition, which assumes that the composition of the liquid phase surrounding a molecule is different from the bulk composition due to molecular interaction energies [
5,
14].
The mathematical formulation of the NRTL model is as follows:
where
is the activity coefficient of component;
is the interaction energy parameter between component
i and
j;
is the non-randomness parameter (typically between 0.2–0.47 for hydrocarbon systems).
Key features of the NRTL model:
- –
Suitable for systems with non-ideal liquid phases (e.g., highly selective solvents like NMP, DMF, ACN).
- –
Accounts for asymmetric interactions between different components.
- –
Provides accurate phase equilibrium predictions for mixtures with strong molecular interactions [
14].
The selection of the NRTL model was critical for correctly simulating the extractive distillation process of butadiene, given the significant non-ideality introduced by the selective solvents used.
The feedstock composition was based on experimental data from the ethane fractionator column, with primary components including butadiene, butenes, and butanes. The experimental data incorporated are shown in
Table 2.
Table 2 presents the gas chromatography results from the ethane fractionator column, listing retention times, peak areas (in pA·s), normalized concentrations (as percentages), and the identified hydrocarbon components. The area under each peak represents the relative amount of each component, while the normalized percentage indicates the proportion of each substance in the sample. Notably, components like Trans-2-Butene and Cis-2-Butene exhibit the highest concentrations, highlighting their prominence in the separation output.
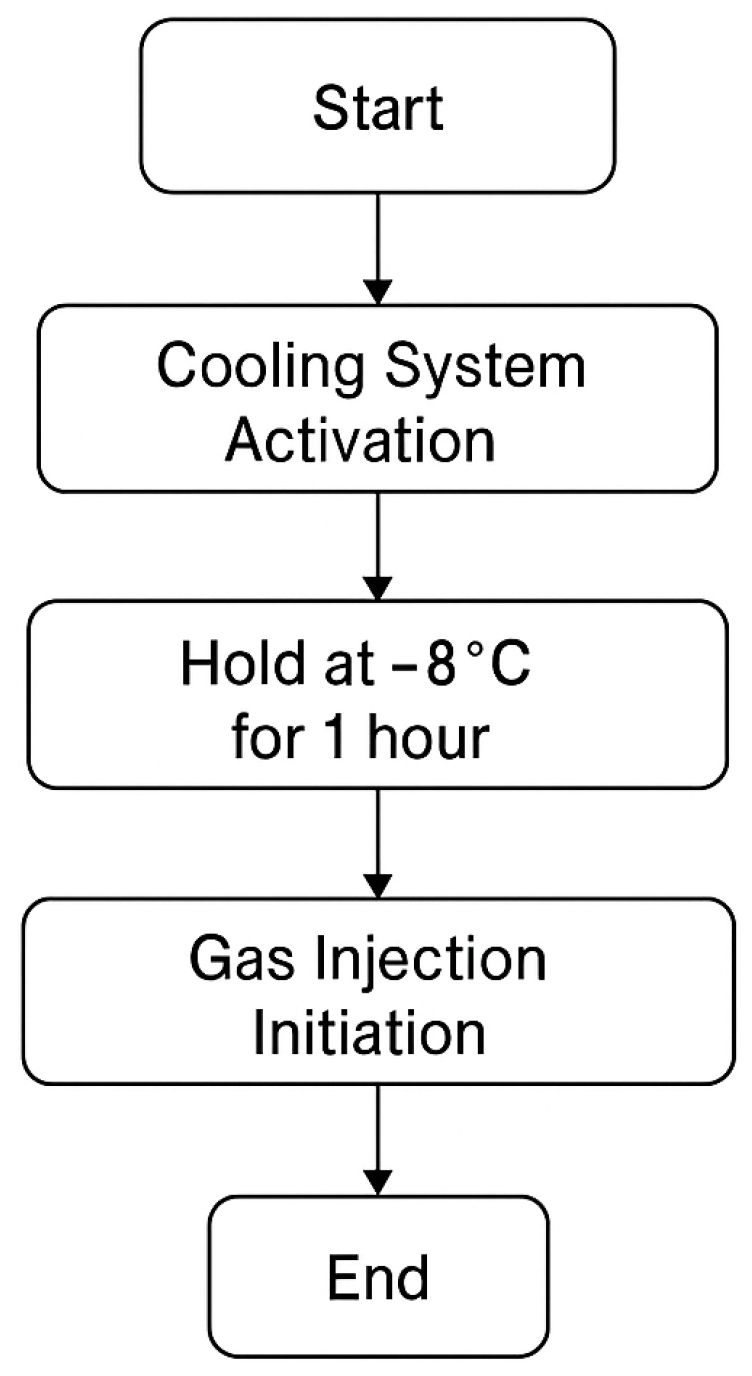
Prior to gas introduction, the cooling system was maintained at −8 °C for one hour. After cooling, gas was injected at a pressure of 3.5 bar for one hour to initiate the experimental validation.
After stabilization, the performance of the separation process was analyzed based on product purity and butadiene recovery efficiency. The optimal conditions identified in the simulation yielded a butadiene recovery rate of 98% with a final product purity of 99.5%. Energy integration between the primary distillation and extractive distillation columns resulted in a 12% reduction in total energy consumption, highlighting the benefits of process optimization. The schematic representation of this procedure is shown in
Figure 3.
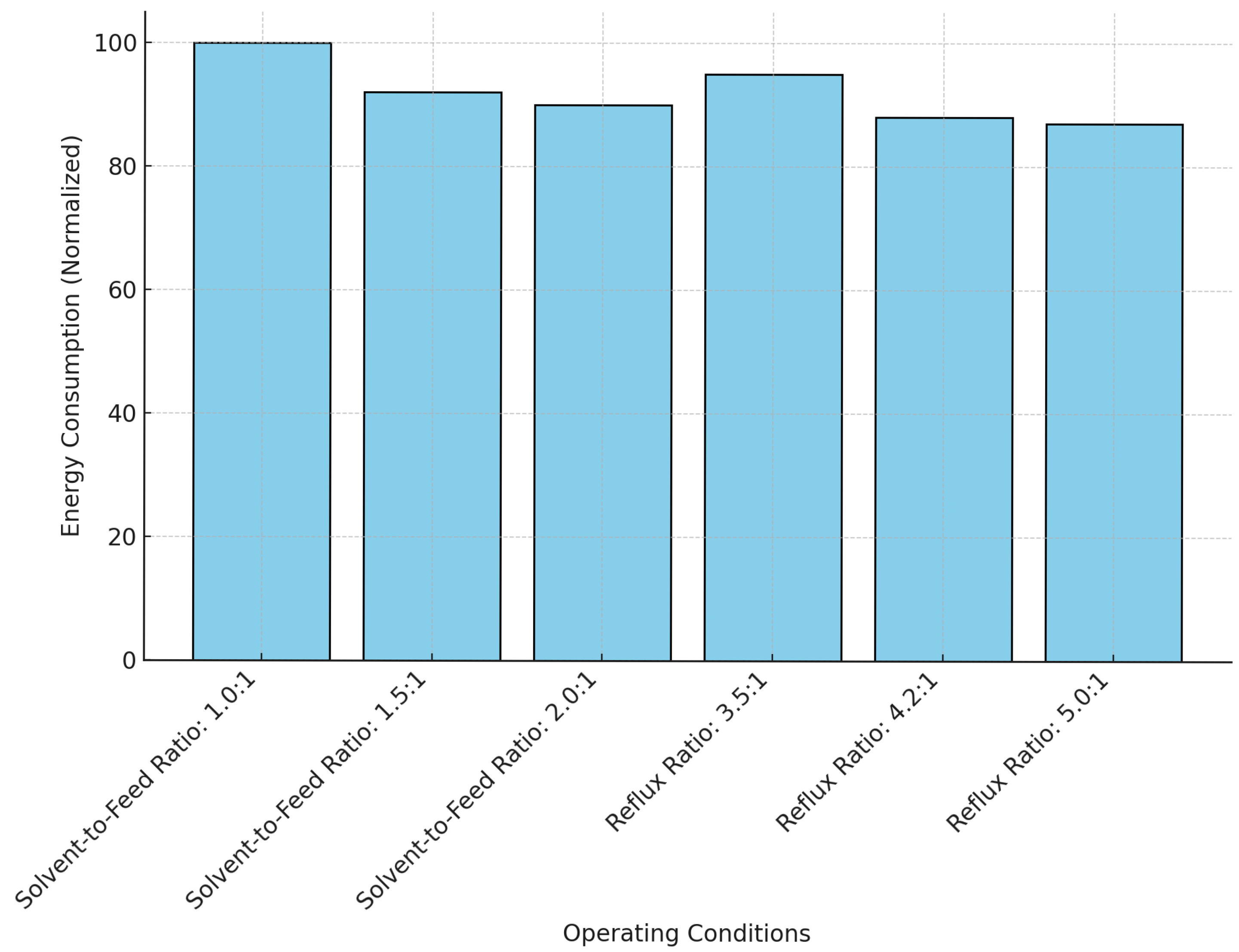
The process parameters—solvent-to-feed ratio, reflux ratio, number of theoretical stages, and heat integration strategies—were selected due to their critical influence on separation efficiency and energy consumption.
Solvent-to-Feed Ratio: Affects butadiene selectivity. Higher ratios improve separation but increase solvent handling and energy costs.
Reflux Ratio: Enhances product purity and recovery but raises energy demand if excessive.
Number of Theoretical Stages:
In distillation processes, a theoretical stage represents a single equilibrium contact between vapor and liquid phases. Increasing the number of theoretical stages improves the separation efficiency by allowing more opportunities for mass transfer between phases [
5]. In practice, a higher number of stages can lead to better butadiene purity and recovery with optimized energy use.
Heat Integration Strategies:
Heat integration refers to the systematic reuse of heat within a process to minimize external heating and cooling requirements. In the butadiene separation process, heat integration strategies include the following:
- –
Preheating the feed stream using the hot streams from overhead vapors;
- –
Heat recovery between the distillation and stripping columns;
- –
Using heat exchangers to transfer energy between different process sections.
Other variables like operating pressure and feed preheating could be explored in future studies to further optimize efficiency. These strategies reduce utility consumption, lower operating costs, and enhance overall process sustainability.
The experimental data used in the simulation were obtained from operational records of the ethane fractionator column at the Shurtan Gas Chemical Complex [
11].
These data include the composition of the C4 fraction, retention times, and concentration profiles, which were measured under controlled industrial conditions.
To ensure the reliability and validity of the data, we took the following steps:
- –
Data acquisition was carried out using calibrated gas chromatography equipment.
- –
Measurements were repeated to confirm consistency.
- –
The obtained data were cross-checked with reported industry-standard values for C
4 hydrocarbon fractions [
11].
Thus, the experimental dataset provides a robust foundation for validating the Aspen Plus model and ensuring its applicability to real-world industrial operations.
To validate the simulation, gas was passed through the ethane fractionator column at a pressure of 3.5 bar. Before gas introduction, the cooling system was maintained at −8 °C for one hour, followed by gas injection at the same pressure for another hour. The results confirmed that the butadiene 1,3 content did not drop below 21%, while heavier hydrocarbons were reduced from 16% to 1%.
The experimental validation described in the study directly corresponds to the operational performance evaluated through the cooling and gas injection procedure.
Specifically, the experimental tests—maintaining the cooling system at −8 °C for 1 h and injecting gas at 3.5 bar for 1 h—were designed to validate the Aspen Plus model predictions on the following:
- –
Butadiene recovery rate;
- –
Product purity;
- –
Energy consumption reduction via heat integration.
The experimental data showed the following:
- –
A butadiene recovery rate of 98%;
- –
A product purity of 99.5%;
- –
Energy savings of approximately 12%, which closely matched the Aspen Plus simulation results (simulation recovery 98.0%, purity 99.5%, energy saving 12.0%).
Thus, the experimental validation confirms that the simulation model is reliable and accurately represents industrial-scale butadiene separation processes.
The performance of the separation process was analyzed based on product purity and recovery efficiency. The optimal conditions identified in the simulation yielded a butadiene recovery rate of 98% with a final product purity of 99.5%. Energy integration between the primary distillation and extractive distillation columns resulted in a 12% reduction in total energy consumption, highlighting the benefits of process optimization.
Experimental validation was conducted to confirm the accuracy of the Aspen Plus model. The results demonstrated good agreement between the simulated and experimental data, supporting the reliability of the model for industrial application.
5. Conclusions
This study highlights the importance of optimizing operational parameters in the butadiene 1,3 separation process. By fine-tuning the solvent-to-feed ratio, reflux ratio, and column design, a high recovery rate of 98% and a product purity of 99.5% were achieved. Additionally, energy integration strategies significantly reduced energy consumption, contributing to a more sustainable and cost-effective separation process. The experimental data confirmed the efficiency of maintaining specific temperature and pressure conditions, ensuring consistent butadiene recovery rates. The findings provide a strong foundation for further research and industrial implementation, improving efficiency in petrochemical separations. The findings provide practical insights for industrial-scale butadiene separation, offering pathways to reduce operational energy costs and enhance process sustainability through optimal parameter tuning and heat integration.
In addition to academic contributions, the findings of this study offer significant practical implications for industrial applications. By optimizing key operational parameters and implementing heat integration strategies, industrial-scale butadiene separation units can achieve substantial reductions in energy consumption and operational costs.
Moreover, the use of advanced simulation and validation methods provides a framework for enhancing process sustainability, supporting the transition towards more energy-efficient and environmentally friendly petrochemical production.