A Comparative Study Using Two Types of Photobioreactor for Cultivation of Chlorella vulgaris Microalgae †
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Methods
2.2.1. Cultivation
2.2.2. Harvesting Biomass
2.2.3. Lipid Extraction
3. Results and Discussions
Lipid Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onay, M.; Sonmez, C.; Oktem, H.; Yucel, M. Evaluation of various extraction techniques for efficient lipid recovery from thermo-resistant microalgae, Hindakia, Scenedesmus and Micractinium species—Comparison of lipid extraction methods from microalgae. Am. J. Anal. Chem. 2016, 7, 141–150. [Google Scholar] [CrossRef]
- Gong, Q.; Feng, Y.; Kang, L.; Luo, M.; Yang, J. Effect of light and pH on cell density of Chlorella vulgaris. Energy Procedia 2014, 61, 2012–2015. [Google Scholar] [CrossRef]
- Aguoru, C.U.; Okibe, P.O. Content and composition of lipid produced by Chlorella vulgaris for biodiesel production Adv. Life Sci. Technol. 2015, 36, 96–100. [Google Scholar]
- Moazami, N.; Ranjbar, R.; Ashori, A.; Tangestani, M.; Nejad, A.S. Biomass and lipid productivities of marine microalgae isolated from the Persian Gulf and the Qeshm Island. Biomass Bioenergy 2011, 35, 1935–1939. [Google Scholar] [CrossRef]
- Mustapa, N.S.; Abu Mansor, M.S.; Serri, N.A. Design and development of centred-light photobioreactor for microalgae cultivation system. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012009. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Blackburn, S.I. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kutz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]
- Anbalagan, L.; Serri, N.A.; Kassim, M.A. Investigation of different environmental conditions: Influence on lipid accumulations by Halochlorella rubescens. AIP Conf. Proc. 2023, 2785, 040004. [Google Scholar]
- Naderi, G.; Tadé, M.O.; Znad, H. Modified photobioreactor for biofixation of carbon dioxide by Chlorella vulgaris at different light intensities. Chem. Eng. Technol. 2015, 38, 1371–1379. [Google Scholar] [CrossRef]
- Al-Qasmi, M.; Talebi, S.; Al-Rajhi, S.; Al-Barwani, T. A review of effect of light on microalgae growth. In Proceedings of the World Congress on Engineering 2012, WCE 2012, London, UK, 4–6 July 2012; Volume I. [Google Scholar]
- Blinová, L.; Bartošová, A.; Gerulová, K. Cultivation of Microalgae (Chlorella vulgaris) for Biodiesel Production; Research Papers; Faculty of Materials Science and Technology, Slovak University of Technology: Bratislava, Slovakia, 2015; Volume 23. [Google Scholar]
- Daliry, S.; Hallajisani, A.; Mohammadi, R.J.; Nouri, H.; Golzary, A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Global J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar]
- Rai, M.P.; Gautom, T.; Shara, N. Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. J. Biol. Sci. 2015, 15, 260–267. [Google Scholar] [CrossRef]
- Minillo, A.; Godoy, H.C.; Fonseca, G.G. Growth performance of microalgae exposed to CO2. J. Clean Energy Technol. 2013, 1, 110–114. [Google Scholar] [CrossRef]
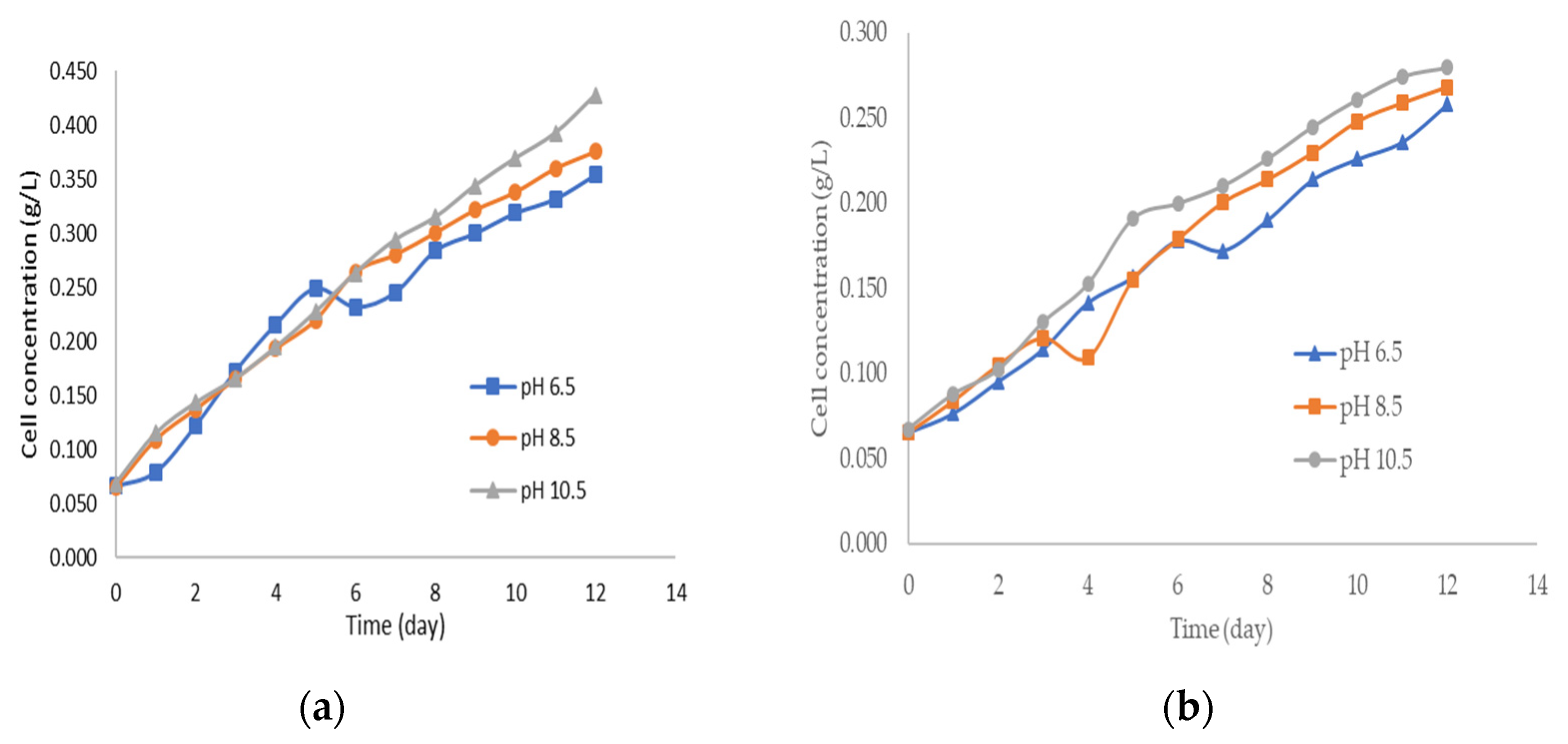
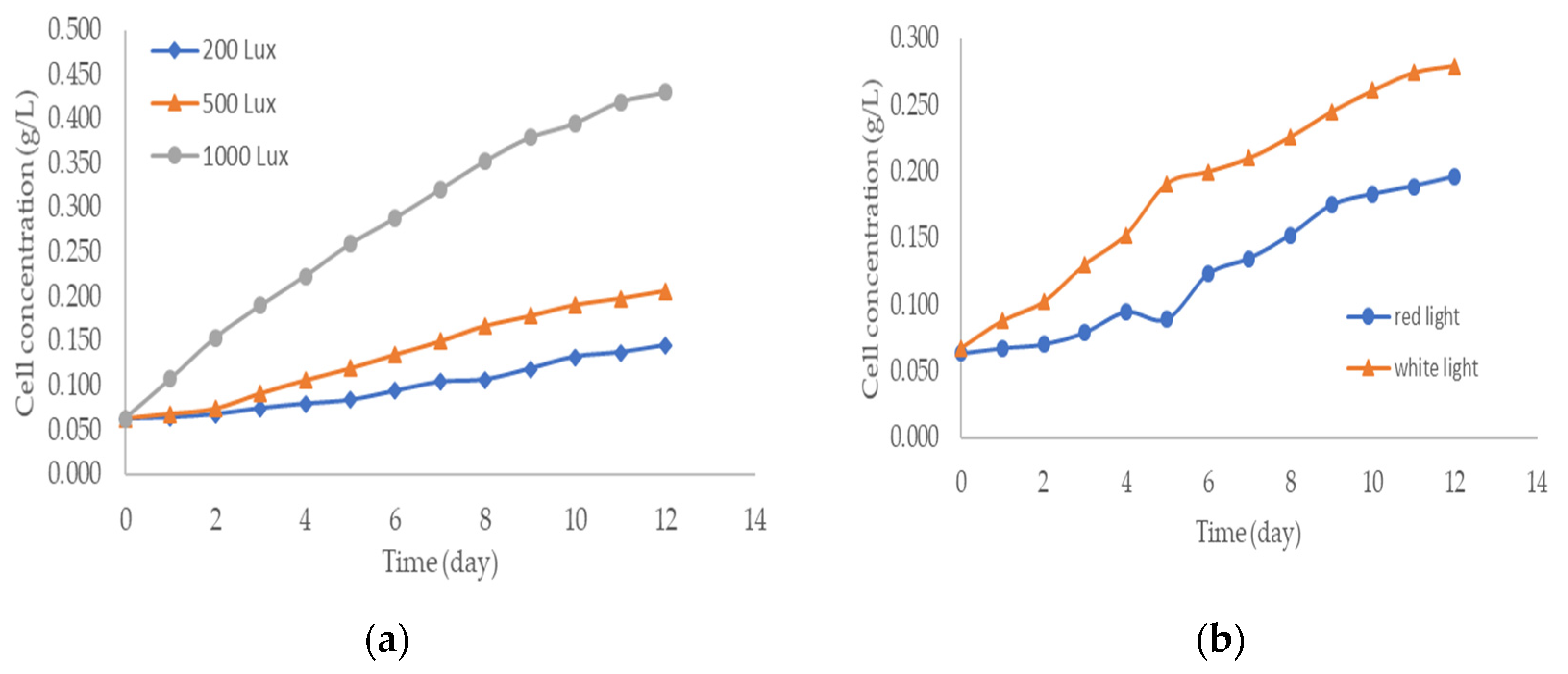
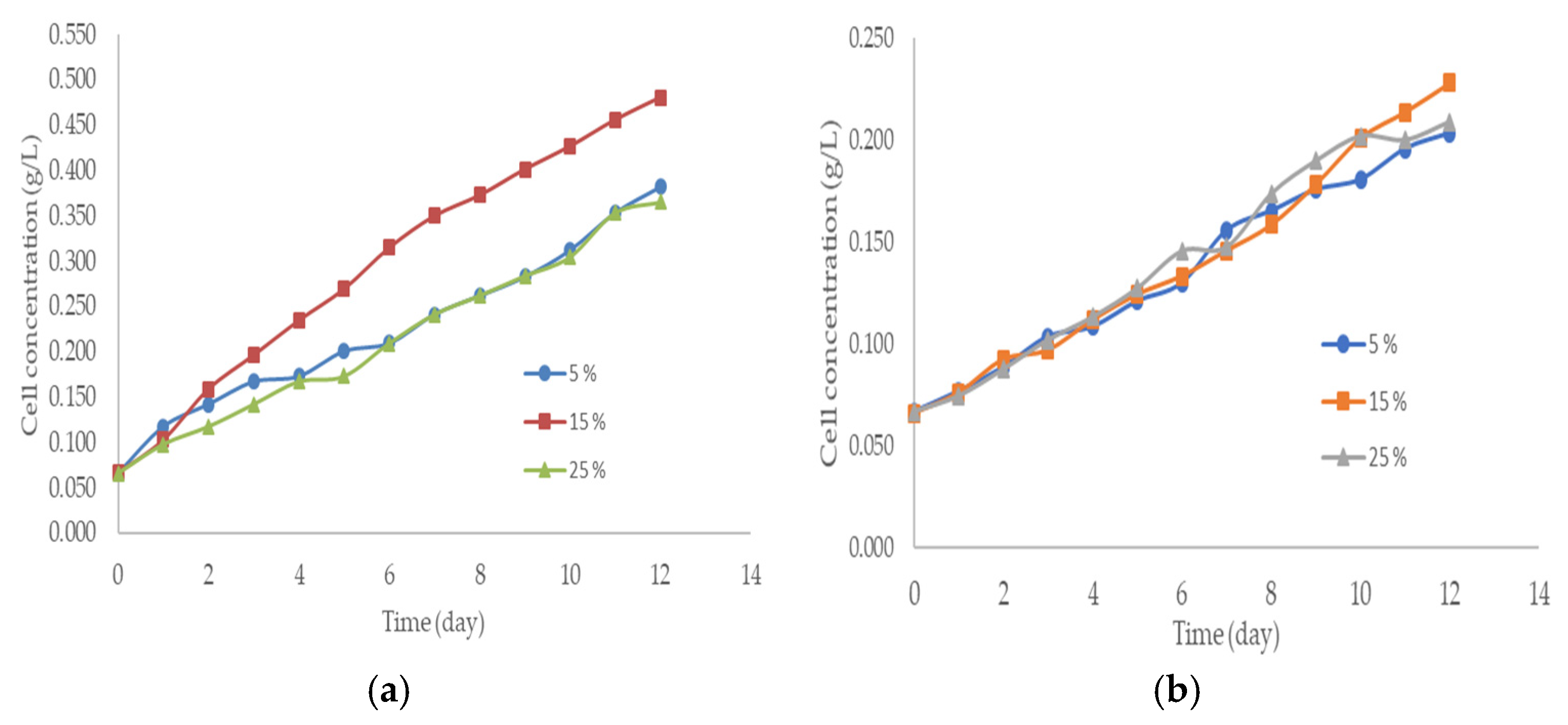
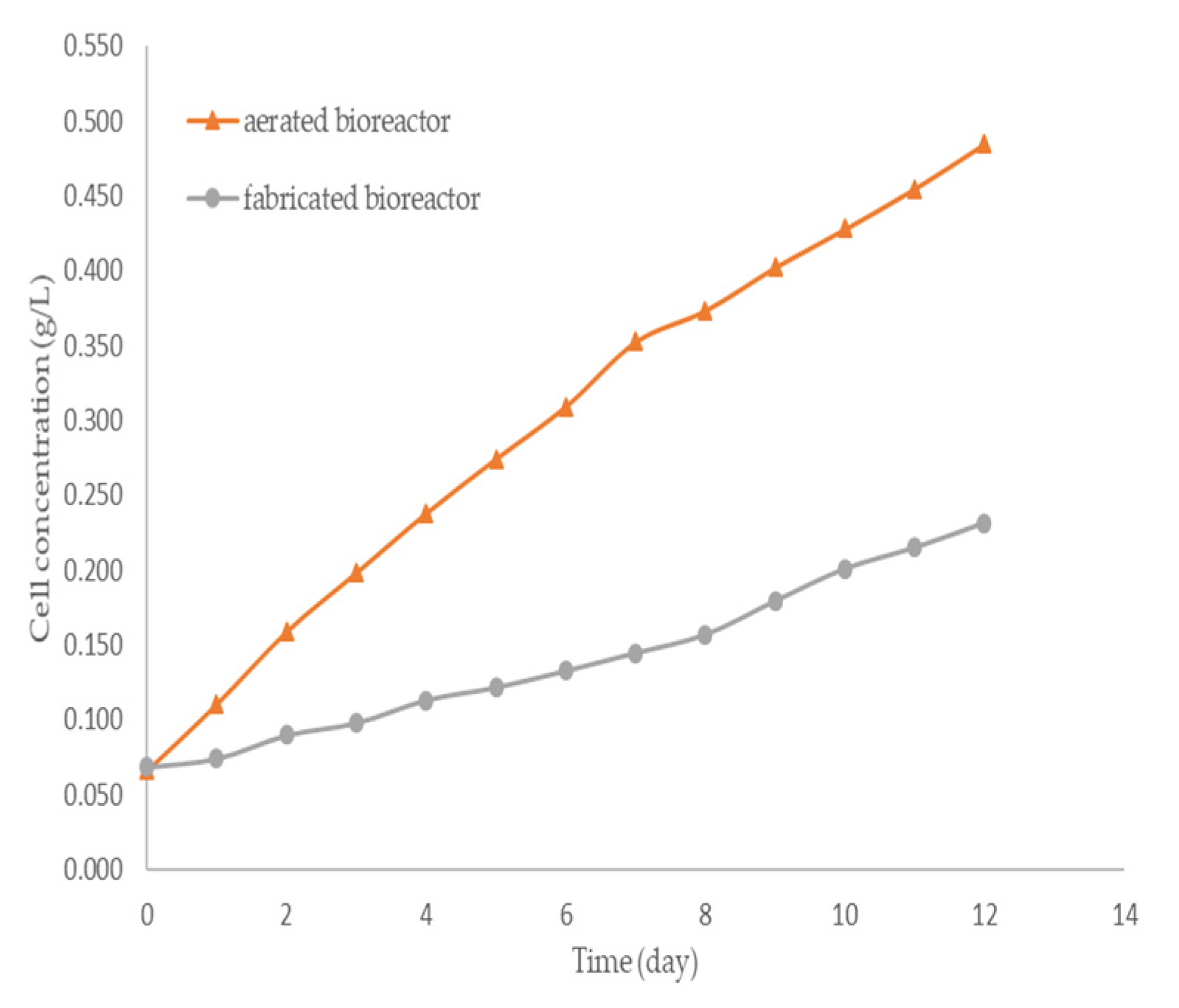
| Factor | Lipid Content (%) | |
|---|---|---|
| pH Medium | Aerated Bottle | Fabricated Reactor |
| pH 6.5 | 18.20 | 13.02 |
| pH 8.5 | 17.12 | 15.05 |
| pH 10.5 | 23.00 | 19.52 |
| Light intensity | ||
| 200 Lux | 17.77 | *na |
| 500 lux | 18.60 | *na |
| 1000 lux | 11.93 | *na |
| White light | *na | 13.94 |
| Red light | *na | 23.00 |
| CO2 concentration (%) | ||
| 5% | 27.63 | 18.63 |
| 15% | 20.07 | 19.53 |
| 25% | 18.06 | 14.09 |
| Optimum conditions pH 10.5, 15% CO2, white light, (1000 lux for aerated vessel) | 21.05 | 20.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serri, N.A.; Halmi, N.H.; Kassim, M.A.; Mansor, M.S.A. A Comparative Study Using Two Types of Photobioreactor for Cultivation of Chlorella vulgaris Microalgae. Eng. Proc. 2025, 84, 6. https://doi.org/10.3390/engproc2025084006
Serri NA, Halmi NH, Kassim MA, Mansor MSA. A Comparative Study Using Two Types of Photobioreactor for Cultivation of Chlorella vulgaris Microalgae. Engineering Proceedings. 2025; 84(1):6. https://doi.org/10.3390/engproc2025084006
Chicago/Turabian StyleSerri, Noor Aziah, Nur Hazwani Halmi, Mohd Asyraf Kassim, and Mohd Salman Abu Mansor. 2025. "A Comparative Study Using Two Types of Photobioreactor for Cultivation of Chlorella vulgaris Microalgae" Engineering Proceedings 84, no. 1: 6. https://doi.org/10.3390/engproc2025084006
APA StyleSerri, N. A., Halmi, N. H., Kassim, M. A., & Mansor, M. S. A. (2025). A Comparative Study Using Two Types of Photobioreactor for Cultivation of Chlorella vulgaris Microalgae. Engineering Proceedings, 84(1), 6. https://doi.org/10.3390/engproc2025084006





