Designing Metal Hydride-Phase Change Material with Analytical and Numerical Method †
Abstract
1. Introduction
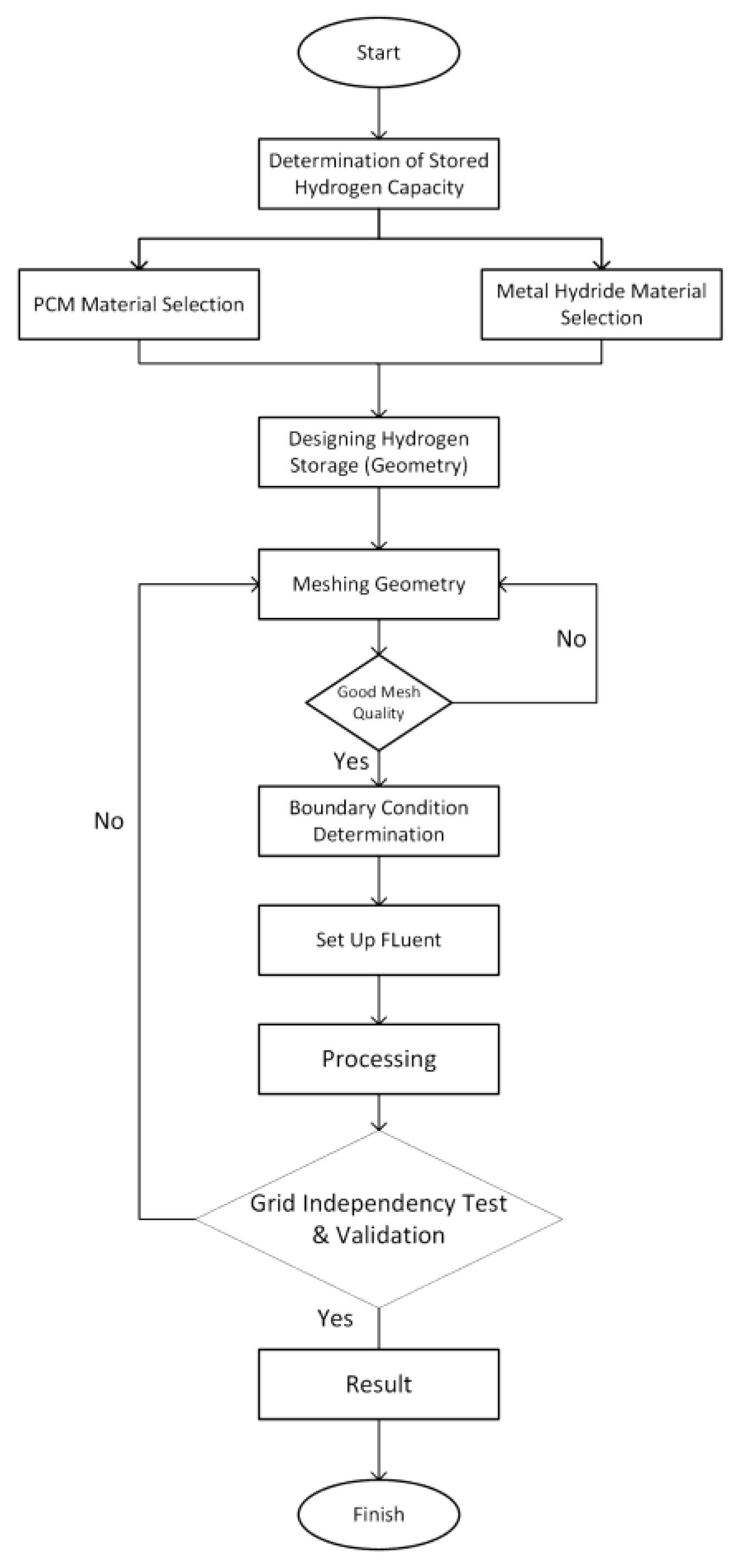
2. Method
2.1. Analytical Method
2.2. Computational Method
3. Result and Discussion
3.1. Volume and Design
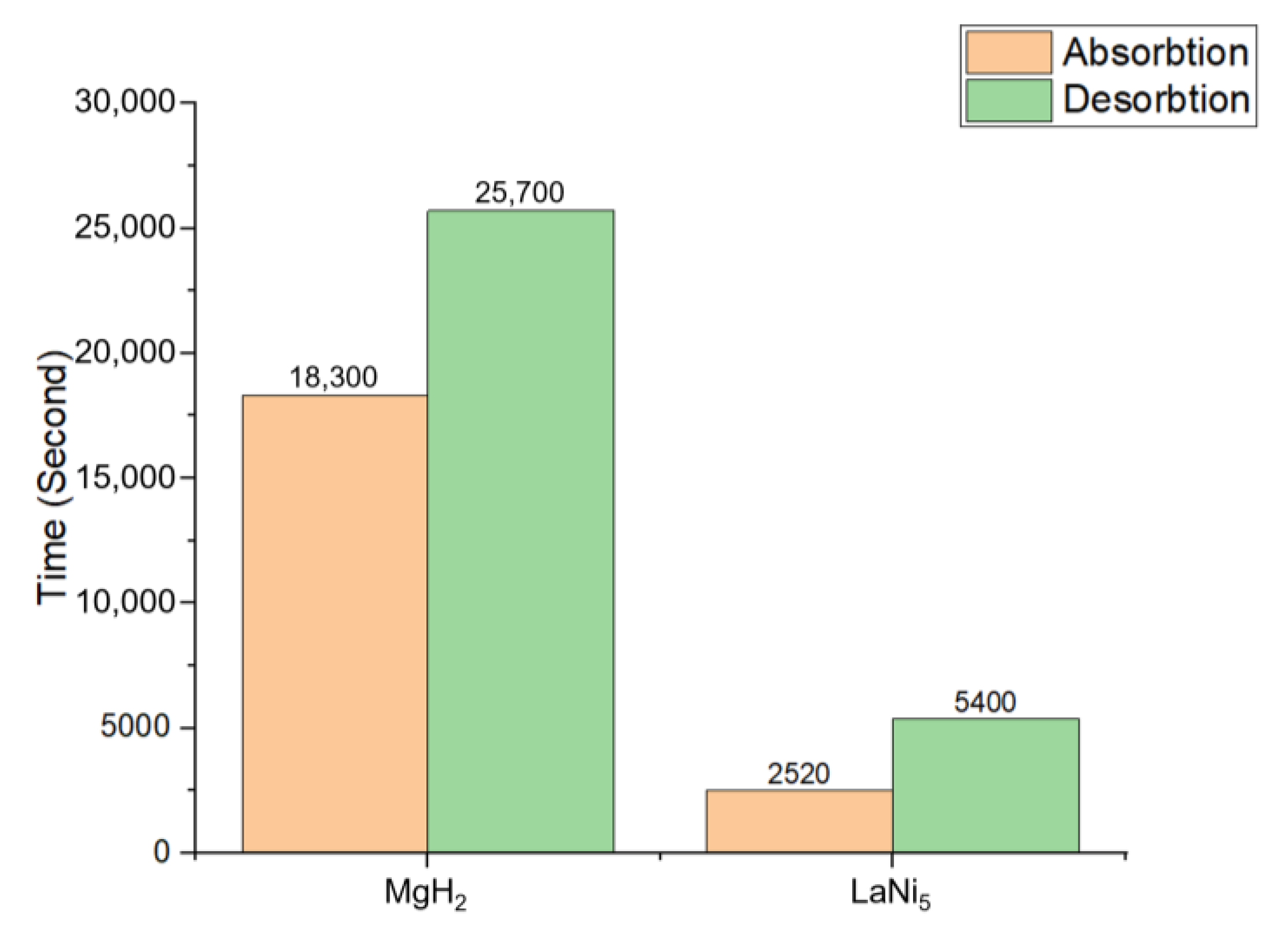
3.2. Time and Temperature
4. Conclusions
- MgH2 necessitates a bigger volume of phase change material (PCM) because of its greater reaction enthalpy, resulting in the need for more effective thermal management compared to LaNi5.
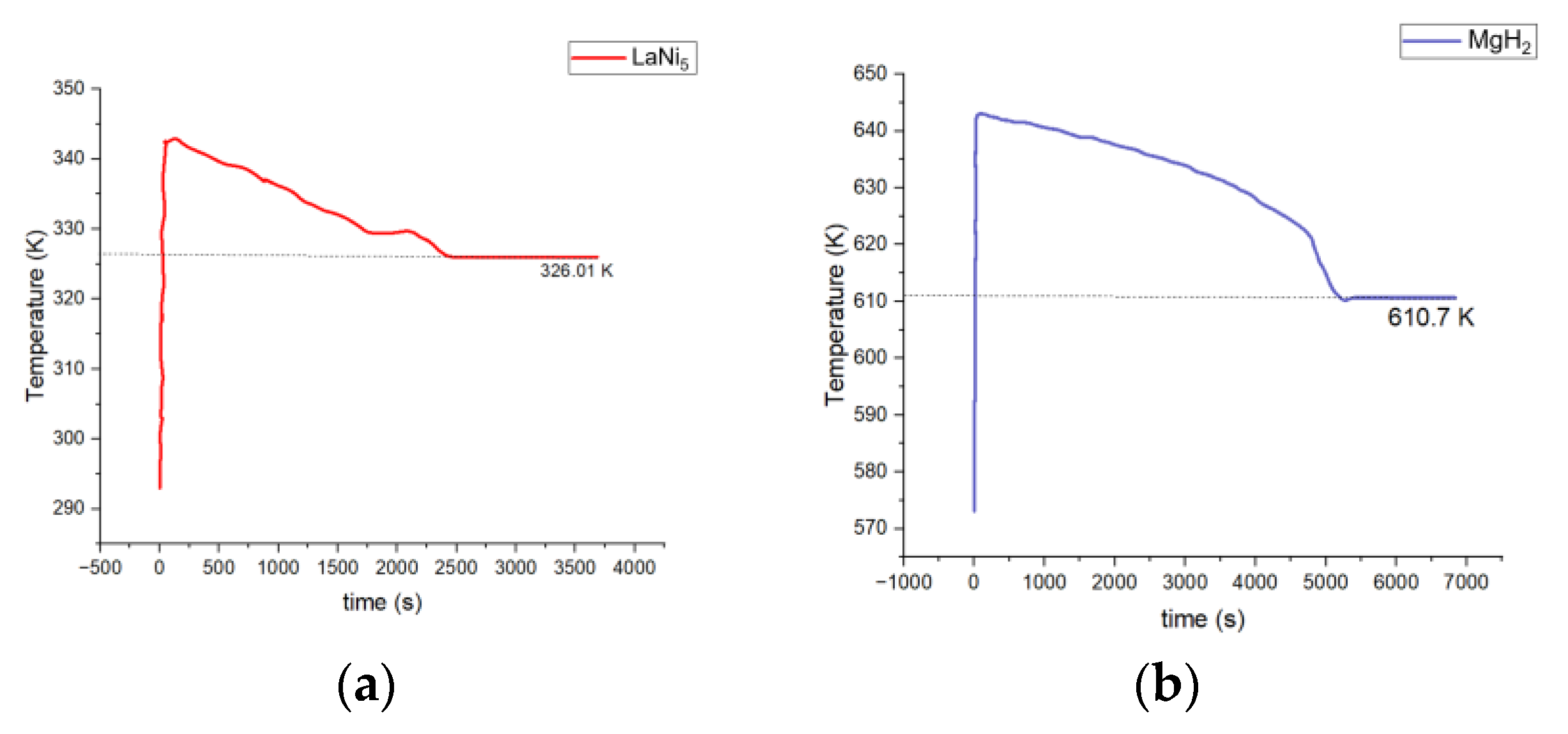
- Magnesium hydride, having slower reaction rates, is more suitable for systems focusing on greater storage capabilities. The operating temperature range for MgH2 is between 610.7 and 643.2 K, which is significantly higher than the 326.01 to 344.5 K operating temperature of LaNi5, indicating that MgH2 is more suitable for applications requiring high temperatures, whereas LaNi5 is more beneficial for moderate-temperature operations. In contrast, MgH2, with slower kinetics, is better suited for systems prioritizing higher storage capacity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, G.; Li, P.; Liu, C.; Liu, Y.; Zhang, H.; Lin, F.; Qu, X. Review of thermal management technology for metal hydride reaction beds. Sustain. Energy Fuels 2023, 7, 2025–2041. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Ye, Y.; Lu, J.; Ding, J.; Wang, W.; Yan, J. Numerical simulation on the storage performance of a phase change materials based metal hydride hydrogen storage tank. Appl. Energy 2020, 278, 115682. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Li, Y.; Teliz, E.; Zinola, F.; Díaz, V. Design of a AB5-metal hydride cylindrical tank for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 33889–33898. [Google Scholar] [CrossRef]
- Ye, Y.; Ding, J.; Wang, W.; Yan, J. The storage performance of metal hydride hydrogen storage tanks with reaction heat recovery by phase change materials. Appl. Energy 2021, 299, 117255. [Google Scholar] [CrossRef]
- Bao, Z.; Wu, Z.; Nyamsi, S.N.; Yang, F.; Zhang, Z. Three-dimensional modeling and sensitivity analysis of multi-tubular metal hydride reactors. Appl. Therm. Eng. 2013, 52, 97–108. [Google Scholar] [CrossRef]
- Bao, Z.; Yang, F.; Wu, Z.; Nyamsi, S.N.; Zhang, Z. Optimal design of metal hydride reactors based on CFD–Taguchi combined method. Energy Convers. Manag. 2013, 65, 322–330. [Google Scholar] [CrossRef]
- Disli, T.; Çetinkaya, S.A.; Ezan, M.A.; Colpan, C.O. Numerical investigations on the absorption of a metal hydride hydrogen storage tank based on various thermal management strategies. Int. J. Hydrogen Energy 2024, 51, 504–522. [Google Scholar] [CrossRef]
- Hariyadi, A.; Suwarno, S.; Denys, R.V.; Von Colbe, J.B.; Sætre, T.O.; Yartys, V. Modeling of the hydrogen sorption kinetics in an AB2 laves type metal hydride alloy. J. Alloys Compd. 2022, 893, 162135. [Google Scholar] [CrossRef]
- Sato, T.; Saitoh, H.; Utsumi, R.; Ito, J.; Nakahira, Y.; Obana, K.; Takagi, S.; Orimo, S.I. Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules 2023, 28, 1256. [Google Scholar] [CrossRef] [PubMed]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Maggini, M. Enhancing Metal Hydride—Phase Change Material Hydrogen Storage Systems Efficiency with Expanded Graphit. Energy Proc. 2024, 49. [Google Scholar] [CrossRef]
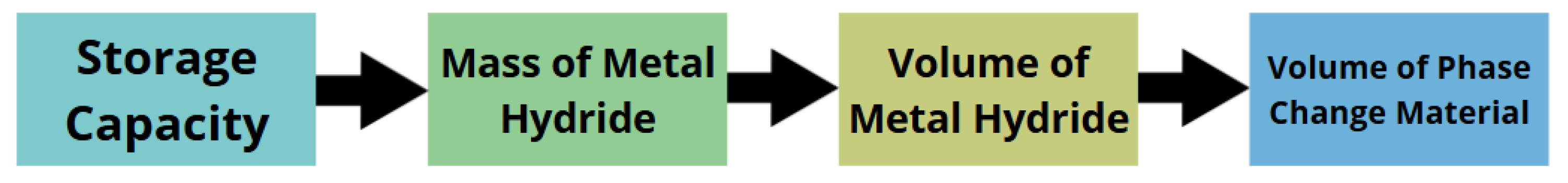
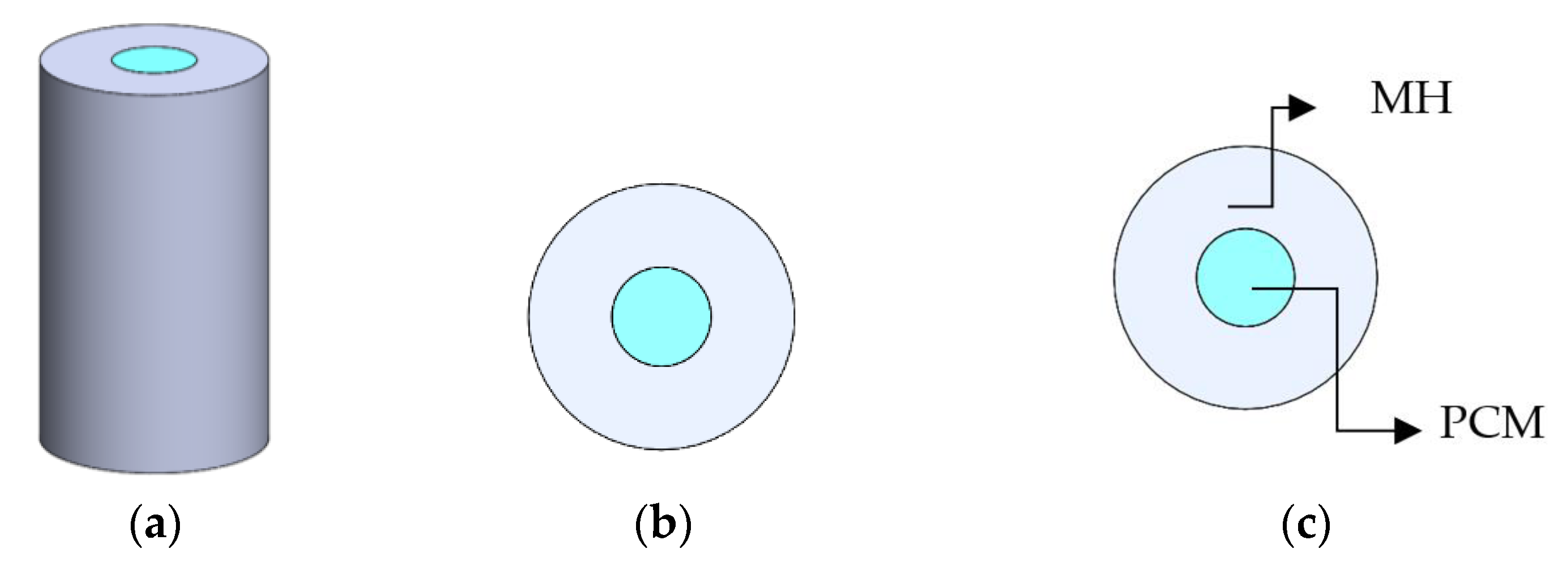
| Material of MH | Hydrogen | Volume of MH | Volume of PCM |
|---|---|---|---|
| MgH2 | 0.3 kg | 0.0023 m3 | 0.00032 m3 |
| LaNi5 | 0.3 kg | 0.0020 m3 | 0.00008 m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, A.T.; Ramadhan, F.; Suwarta, P.; Witantyo, W.; Suwarno, S. Designing Metal Hydride-Phase Change Material with Analytical and Numerical Method. Eng. Proc. 2025, 84, 51. https://doi.org/10.3390/engproc2025084051
Rahayu AT, Ramadhan F, Suwarta P, Witantyo W, Suwarno S. Designing Metal Hydride-Phase Change Material with Analytical and Numerical Method. Engineering Proceedings. 2025; 84(1):51. https://doi.org/10.3390/engproc2025084051
Chicago/Turabian StyleRahayu, Ajeng Tri, Faizal Ramadhan, Putu Suwarta, Witantyo Witantyo, and Suwarno Suwarno. 2025. "Designing Metal Hydride-Phase Change Material with Analytical and Numerical Method" Engineering Proceedings 84, no. 1: 51. https://doi.org/10.3390/engproc2025084051
APA StyleRahayu, A. T., Ramadhan, F., Suwarta, P., Witantyo, W., & Suwarno, S. (2025). Designing Metal Hydride-Phase Change Material with Analytical and Numerical Method. Engineering Proceedings, 84(1), 51. https://doi.org/10.3390/engproc2025084051





