Abstract
This paper adds to the sustainable materials field by in-situ epoxidation of rubber seed oil (RSO), a highly underutilized resource that has been sporadically used, using an optimized combination of 30% hydrogen peroxide and acetic/formic acid sulfuric acid. Most of the previous studies deal with more common vegetable oils, where the main focus in most is given to the epoxidation of these oils and their derivatives. The RSO contained a high iodine value around 135.36 g–I2/100 g. The central to this work is the systematic study of the oxirane number as a function of reaction temperature and the double bond:RCOOH:H2O2 molar ratios. By testing the temperatures of 40, 50, 60, and 70 °C and three specific molar ratios (1:0.6:1.4, 1:1:2, and 1:1.5:3), this research not only found the optimal conditions for epoxidation but also gave valuable information on the reaction kinetics of rubber seed oil. The results showed that a temperature of 60 °C with a 1:1:2 molar ratio gave the highest oxirane number, especially with performic acid, which was 3.200 mmol/g. Then, overall, formic acid consistently outperforms acetic acid in terms of product yields, facilitating a more effective epoxidation process.
1. Introduction
The growing interest in sustainable and green-based materials has encouraged the potential of using renewable resources, particularly plant oils, as substitutes for petroleum materials [1,2]. Among numerous oils, the rubber tree’s rubber seed oil, Hevea brasiliensis, is uniquely and necessarily advantageous depending on natural availability and differing molecular structure. Furthermore, the properties of the oils can be significantly improved by performing reactions based on epoxidation, which will significantly broaden the class of applications (bioplastics to coatings) for which processing is feasible. The use of epoxidation under conditions that have been measured in time and safety simply becomes a bonus of using peroxyacids as an in-situ reaction for renewable resources [3].
The in-situ epoxidation of vegetable oils has emerged as a primary area of research for renewable resources based on oils, specifically including rubber seed oil. These oils are chemical feedstock for the unsaturated fatty acids contained in can be effectively epoxidized that ultimately improve the oils’ chemical character and facilitate radically novel applications. Generally, peroxyacids will be the reactant for the addition process and established that efficacy will apply mild conditions [4,5,6]. Further studies are attached to optimizing epoxidation processes toward an efficient, safer, and sustainable methodology of future sustainability through decreasing risk and enhancing the controllability for safety issues. In particular, there are studies on conducting temperature and molar ratios of the reactants, i.e., the feedstock double bonds, carboxylic acids (RCOOH), and hydrogen peroxide (H2O2). Previous studies have demonstrated that increasing cooking temperature variables increases the rates of conversion by increasing the rate of reaction variables, but taking the temperature too high could also increase undesired side reactions and thus decrease the chemical selectivity and overall yield [7,8,9,10]. Peroxyacids are preferential for selective epoxidation by the introduction of oxirane groups in unsaturated fatty acids. A recent study also used different carboxylic acids: acetic, formic, and other fatty acids in the optimization of epoxidation. The carboxylic acids were found to have influenced the kinetics of the epoxidation reaction [8,11].
This paper is a significant contribution to the field of green materials, with a focus on the in-situ epoxidation of rubber seed oil with peroxyacids with an investigation and optimization on reaction parameters. Unlike recent works by other researches, which focused more on the epoxidation of vegetable oils extracted predominantly from plant sources that are used in synthetic rubber production, our study casts a different perspective, considering the properties of rubber seed oil that were less understood, despite the enrichment of unsaturated fatty acid in this material. In this study, we first investigate the effect of temperature, the type of carboxylic acids, the molar ratio of double bonds, carboxylic acids (RCOOH), and hydrogen peroxide (H2O2) related to conversion. We then optimize these three reaction parameters to maximize the conversion and, in a more delicate way, address the problem of selectivity and minimize the by-products formed as discussed in the previous epoxidation procedures. The molar ratio of the reactants, crucial to our investigation, contributes much to the conversion. For example, compared to those in the previous study working under similar experimental conditions, we explore styles to increase the amount of RCOOH, which stabilizes the reaction media and increases both production of epoxy group and reduction in the other two by-products obtained from the rubber seed oil.
2. Materials and Methods
2.1. Materials
Rubber seed oil (RSO) was purchased directly from local farmers. RSO was degummed and purified using phosphoric acid. The researchers used a variety of chemicals, including analytical grade glacial acetic acid, potassium hydrogen phthalate, Wijs solution, 98% sulfuric acid, 30% aqueous hydrogen peroxide, potassium iodide, 47% hydrobromic acid in acetic acid, and crystal violet indicator.
2.2. Epoxidation Process
In a three-neck flask, a predetermined amount of rubber seed oil (70 g) was mixed with varying amounts of carboxylic acids (such as acetic or formic acid) and hydrogen peroxide, following specific molar ratios of double bond:RCOOH: H2O2 (e.g., 1:0.6:1.4, 1:1:2, and 1:1.5:3). The mixture was constantly mixed and heated to various temperatures (40 °C, 50 °C, 60 °C, and 70 °C) while the reaction was allowed to continue for specified time intervals (0, 30, 60, 120, and 240 min). Samples were taken at each time point for spectroscopic analysis, typically using FTIR, to assess the conversion rates of double bonds and the formation of epoxide groups, providing insights into the optimization of the epoxidation process.
3. Results and Discussion
3.1. Temperature Influence
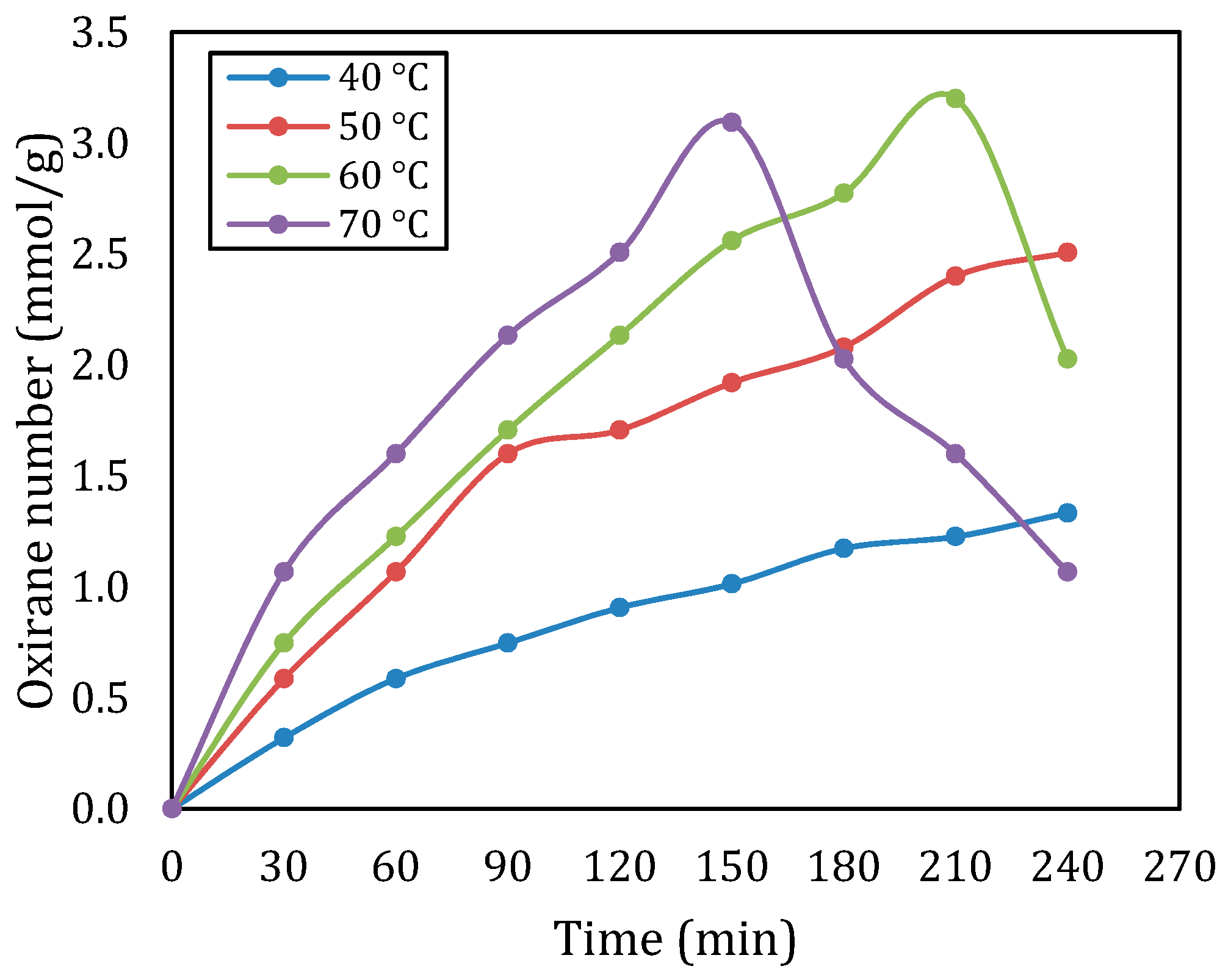
Figure 1 describes the oxirane number of epoxidized rubber seed oil at various temperatures for a molar ratio of double bond:CH3COOH: H2O2 of 1:1:2. The findings showed that the oxirane number was clearly affected by the changes in temperature. For all temperatures, at a certain reaction time (up to 150 min), an increase in temperature will increase the oxirane number. At temperatures of 40 °C and 50 °C, the oxirane number increases continuously from the beginning of the reaction to the end of the reaction, with the optimum oxirane number being 1.333 mmol/g and 1.507 mmol/g, respectively. This implies that the increased temperatures support the reaction rate for epoxidation processes and facilitate a quicker generation of oxirane units. When the temperature was set at 60 °C, the optimum oxirane value was obtained at a reaction time of 210 min, namely 3200 mmol/g. After this time, the oxirane number decreased. This tendency also occurs at a temperature of 70 °C, the oxirane number increases with increasing reaction time (up to 150 min) until the highest point is 3.093 mmol/g, after which the oxirane number decreases drastically. A trend consistent with observations from previous studies showed that higher temperatures (above 60 °C) can have an adverse effect on oxirane number. This decrease in oxirane number could be due to the fact that oxirane is very reactive, so it easily undergoes further reactions with the remaining reactants. This statement is in line with previous research findings [7,10,12]. Overall, the highest oxirane number was obtained at a temperature of 60 °C, namely 3200 mmol/g.
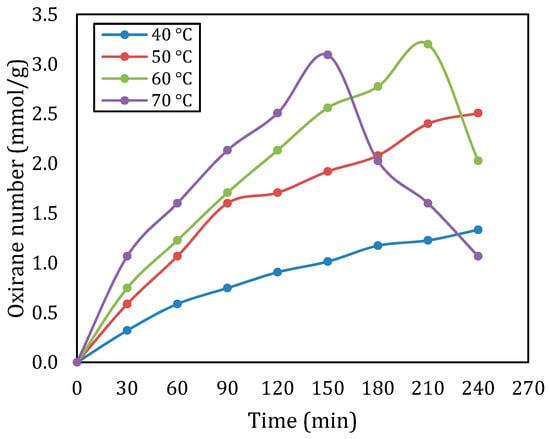
Figure 1.
Oxirane number at various temperatures for a molar ratio of double bond:CH3COOH:H2O2 of 1:1:2.
The data also show that the oxirane number increases over time in a time manner. There was a significant rise in conversion particularly within the 30 min, at temperatures of 50 °C and above. However, the oxirane number tended to level off after 150 min at 70 °C with values declining, suggesting a point of saturation where extended reaction times result in diminishing returns. The plateau aligns with the research by Budiyati et al. (2024), indicating that extended response times may decrease effectiveness by causing the creation of byproducts or the deterioration of the epoxide structure [13].
When comparing these findings to existing literature, several trends become evident. Many previous studies have documented optimal temperature ranges for epoxidation processes, typically around 50–60 °C. The drastic decline in oxirane number observed at 70 °C in this study inlines with some reports where adverse effects were noted at similar temperatures [7,13,14].
3.2. Molar Ratio Influence
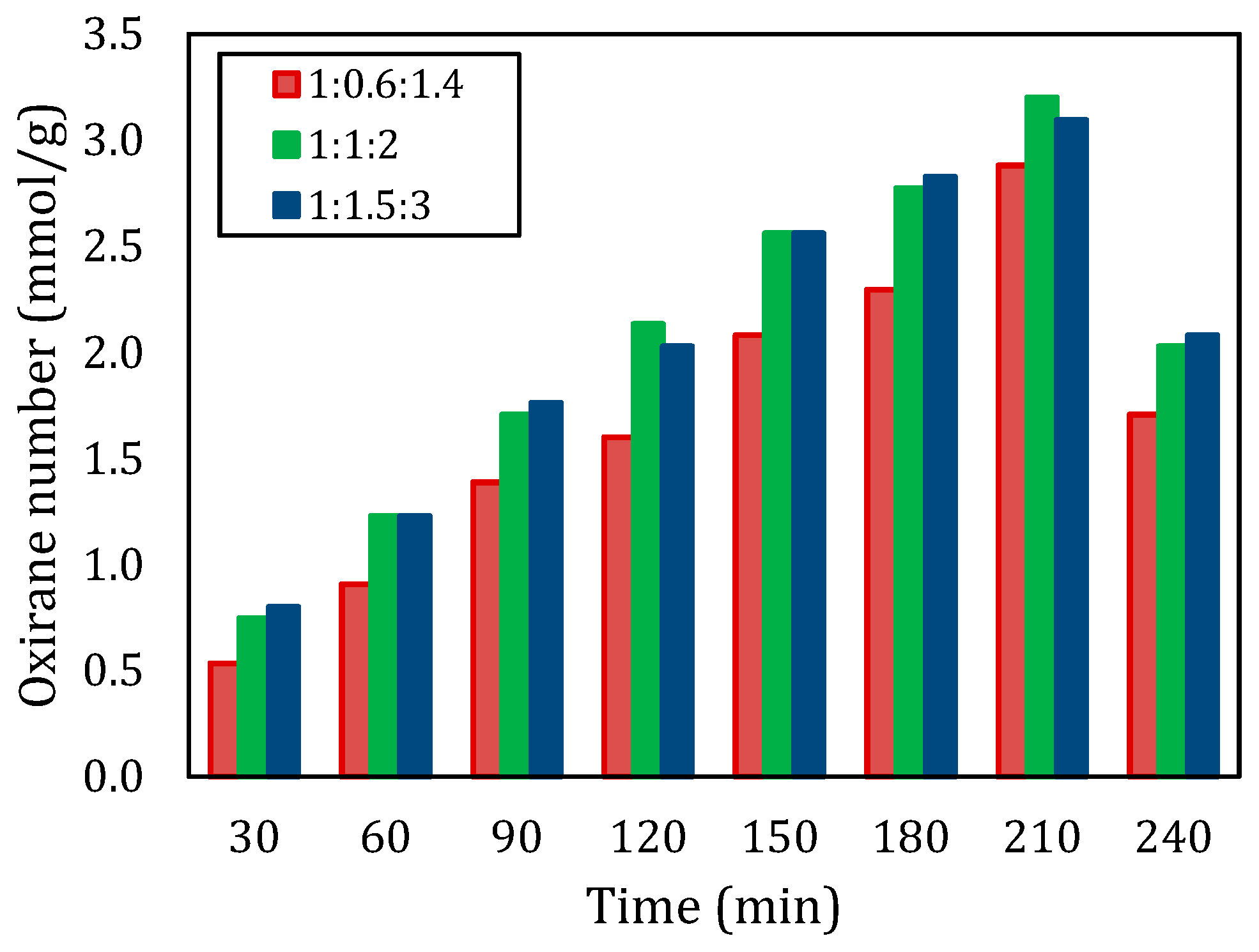
Figure 2 illustrates the oxirane number at various molar ratios for a temperature of 60 °C. The type of carboxylic acid is acetic acid (CH3COOH). The molar ratios of reactants had an impact on how the reactions turned out for this experiment we did with epoxidation processes at different temperatures and time intervals. We found that using the ratio of 1:1:2 consistently gave us results compared to other ratios we tested. The oxirane number for the 1:1:2 ratio came out to be around 3.200 mmol/g, while the 1:0.6:1.4 ratio only reached 2.880 mmol/g. This indicates that having a high mole of CH3COOH and H2O2 in a ratio of 1:1:2 improves the reaction conditions for epoxidization and underscores the significance of maintaining stoichiometric balance in this process. Past research has consistently demonstrated that increasing the proportions of RCOOH and H2O2 leads to conversion rates, a finding that is well supported by the results of this study [8,15]. On the other hand, overall, the oxirane number produced at a molar ratio of 1:1:2 was slightly higher than at a molar ratio of 1:1.5:3. These small differences are telling the possibility of (1) the oxirane number approaching the maximum value, and (2) an increase in molar ratios from 1:1:2 to 1:1.5:3 did not have a significant effect. Therefore, it can be concluded that 1:1:2 was the optimum molar ratio.
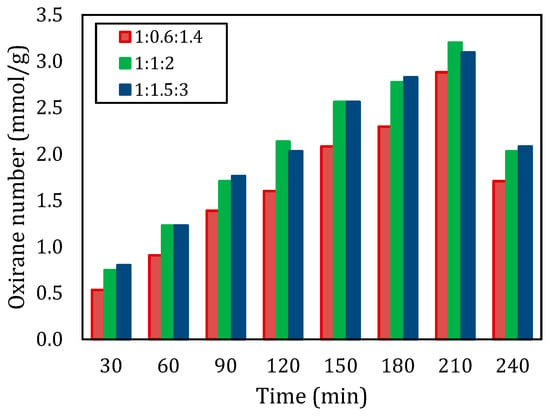
Figure 2.
Oxirane number with different molar ratios of double bond:CH3COOH:H2O2 at a temperature of 60 °C.
3.3. Influence of the Type of Carboxylic Acid
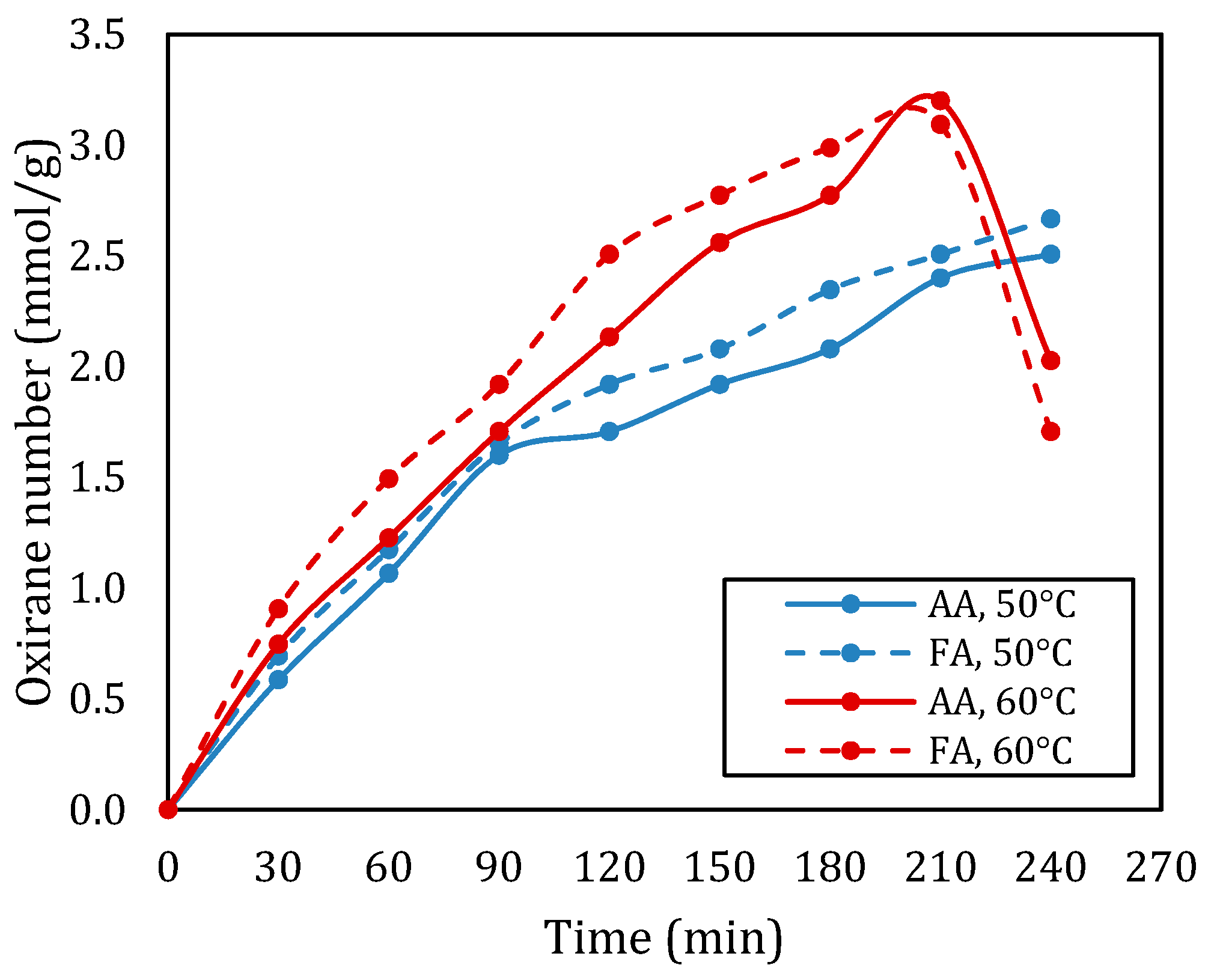
Figure 3 shows the effect of the type of carboxylic acid on the oxirane number in the epoxidation of rubber seed oil. From Figure 3, it can be seen that at a temperature of 50 °C, the oxidation number of rubber seed oil based epoxy using acetic acid is consistently lower than that using formic acid. Meanwhile, at a temperature of 60 °C, formic acid provides a higher oxirane number up to a reaction time of 180 min. After this time, the oxirane value with formic acid decreased significantly, the value was even lower than the oxirane value of epoxy produced from epoxidation of rubber seed oil with acetic acid. The highest oxirane number obtained in epoxidation with formic acid and acetic acid were 3.093 mmol/g and 3.200 mmol/g, respectively.
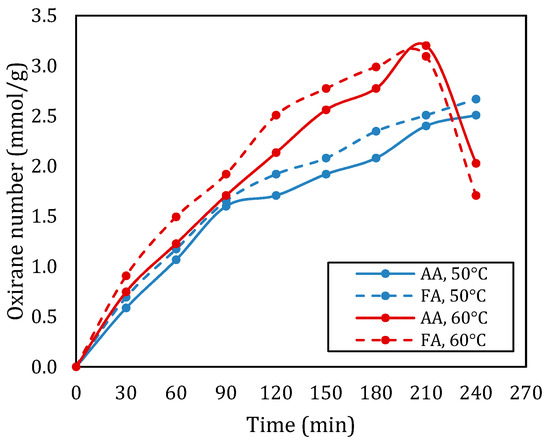
Figure 3.
Oxirane number with different types of acid for the molar ratio of double bond:RCOOH:H2O2 of 1:1:2 at temperatures of 50 °C and 60 °C.
The type of carboxylic acid used in the epoxidation of rubber seed oil significantly impacts the conversion rates and the overall efficiency of the reaction. In this context, formic acid and acetic acid were examined to understand their respective influences on the epoxidation process. When formic acid is employed, it exhibits high reactivity due to its strong oxidizing characteristics. The introduction of peroxyformic acid leads to a rapid conversion of double bonds in rubber seed oil, resulting in higher epoxide yields. Studies have shown that reactions utilizing formic acid can achieve higher conversion within shorter reaction times, particularly at optimized temperatures. The effective formation of reactive intermediates allows for a swift introduction of oxirane groups, making formic acid a superior choice for enhancing the efficiency of the epoxidation process [8,14].
3.4. The FTIR Spectroscopy Data
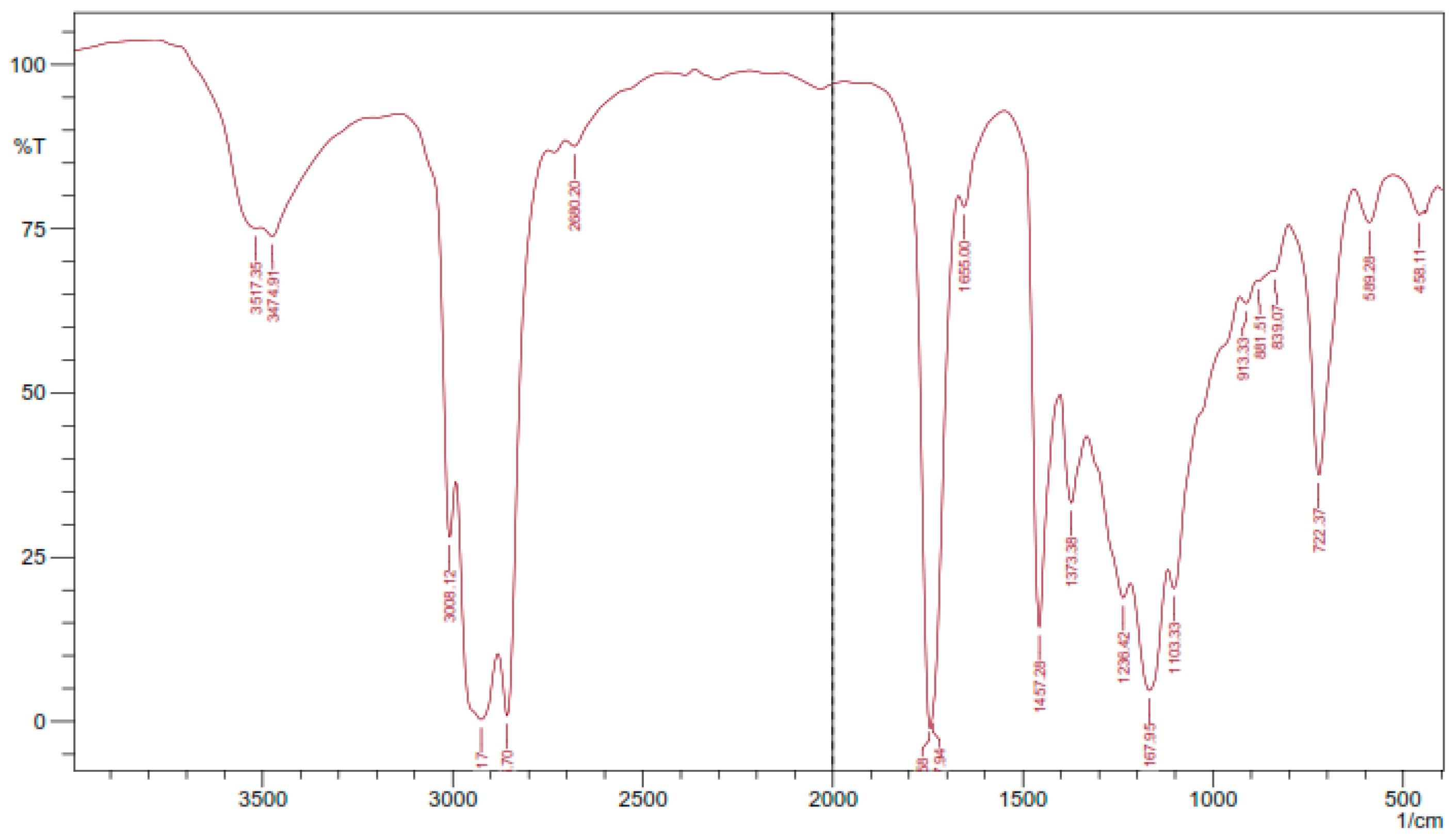
Figure 4 presents the result of FTIR analysis of epoxidized rubber seed oil. The examination of FTIR data for the epoxidation process of rubber seed oil offers insights into the molecular transformations that take place during the reaction process. The presence of absorption bands in the spectrum is linked to functional groups identified in the oil structure and aids in a thorough comprehension of its structural alterations. The FTIR analysis highlights absorption bands that signify the existence of functional groups, within the epoxidized rubber seed oil. The detection of O–H stretching vibrations, between 3474.91–3571.35 cm−1, signals the presence of hydroxyl groups formed during the epoxidation process—an observation, with the research conducted by Budiyati et al. focusing on tung seed oil epoxidation [9], and by Nwosu-Obieogu et al. studying melon seed oil properties [16].
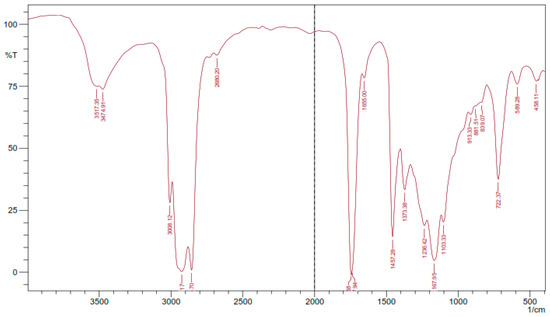
Figure 4.
FTIR analysis results.
The absorption bands linked to C–H stretching at 2925.17 cm−1 and 2856. 7 cm−1 indicate the existence of hydrocarbons. These bands are also supported by the peak at 1457.28 cm−1, which strengthens the idea that aliphatic structures are common in the product. The C = O stretching identified between 1737.94 cm−1 and 1747.58 cm−1 hints, at the presence of carboxylate groups originating from the fatty acids found in rubber seed oil, a discovery that suggests partial oxidation of these functional groups during epoxidation. The absorption peak, at 1167.95 cm−1 corresponding to C–O bonds, provides evidence for the production of alcohols commonly found in epoxidized oils. The detection of C–O–C bonds in the range of 839.07 to 881.51 cm−1 affirms the presence of epoxy groups in the structure, indicating the successful incorporation of oxirane rings into the fatty acid chains. The formation of hydroxyl and epoxy groups in the FTIR spectra of epoxidized vegetable oils reinforces the idea that such modifications are common across different feedstocks. This study’s specific absorption values fall within the ranges reported in the literature, further validating the consistency of FTIR as an analytical tool for monitoring chemical modifications in oils [17].
4. Conclusions
The in situ epoxidation process on rubber seed oil can be carried out well. This study discusses the influence of several important parameters in epoxidation, namely: temperature, time, and molar ratio of double bond:RCOOH: H2O2. The research results showed that epoxidation with performic acid at a reaction temperature of 60 °C and a molar ratio of 1:1:2 produced optimum conversion. The choice of the type of carboxylic acid (formic acid or acetic acid) for epoxidation of rubber seed oil has significant implications for the resulting conversion. Formic acid consistently outperforms acetic acid in terms of reaction kinetics and product yield, thereby facilitating a more effective epoxidation process.
Author Contributions
Conceptualization, E.B.; methodology, E.B. and A.R.; validation, E.B., A.R. and N.A.F.; formal analysis, A.R. and N.A.F.; investigation, E.B.; resources, A.R.; data curation, N.A.F.; writing—original draft preparation, E.B. and A.R.; writing—review and editing, E.B.; visualization, E.B. and N.A.F.; supervision, E.B.; project administration, E.B., A.R. and N.A.F.; funding acquisition, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This material and financial is supported by “Hibah PID”, the research grant from Universitas Muhammadiyah Surakarta.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this paper.
Conflicts of Interest
The authors declare that they have no known competing financial interests orpersonal relationships that could have appeared to influence the work reported in this paper.
References
- Anggono, A.D.; Darmawan, A.S.; Wijianto; Prasojo, C. Development of biodegradable plastic made from recycling of polypropylene (PP) with corn stalks powder. IOP Conf. Ser. Mater. Sci. Eng. 2019, 673, 012136. [Google Scholar] [CrossRef]
- Hidayati, N.U.R.; Mujiburohman, M.U.H.A.M.M.A.D.; Hamid, H.; Purnama, H.E.R.R.Y.; Dwilaksita, A.; Zubaida, F.R. Preliminary study of abs/chitosan blend polymer for dmfc membranes. Mater. Sci. Forum 2019, 961, 23–29. [Google Scholar] [CrossRef]
- Zeng, Y.; Shang, Z.; Zheng, Z.; Shi, N.; Yang, B.; Han, S.; Yan, J. A Review of Chemical Modification of Vegetable Oils and Their Applications. Lubricants 2024, 12, 180. [Google Scholar] [CrossRef]
- Obanla, O.; Udonne, J.; Ajani, O.; Ojewumi, M.; Omodara, O.; Oni, B. Studies of the In-Situ Epoxidation of Rubber (Hevea Brasiliensis)Seed Oil by Performic Acid. J. Phys. Conf. Ser. 2019, 1378, 022025. [Google Scholar] [CrossRef]
- Nwosu-Obieogu, K.; Umunna, M. Rubber Seed Oil Epoxidation: Experimental Study and Soft Computational Prediction. Ann. Fac. Eng. Hunedoara-Int. J. Eng. 2021, 4, 65–70. [Google Scholar]
- Putra, N.R.; Aziz, A.H.A.; Rizkiyah, D.N.; Yunus, M.A.C.; Alwi, R.S.; Qomariyah, L. Green Extraction of Valuable Compounds from Rubber Seed Trees: A Path to Sustainability. Appl. Sci. 2023, 13, 13102. [Google Scholar] [CrossRef]
- Budiyati, E.; Budhijanto; Budiman, A.; Rochmadi. Kinetic study of epoxidation of Tung oil (Reutealis trisperma (Blanco) Airy Shaw) by peroxyacetic acid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012048. [Google Scholar] [CrossRef]
- Budiyati, E.; Rochmadi. The In-situ Epoxidation of Tung Oil by Performic Acid. AIP Conf. Proc. 2024, 2838, 020017. [Google Scholar] [CrossRef]
- Budiyati, E.; Rochmadi, R.; Budiman, A.; Budhijanto, B. Effects of the Molar Ratio of Acetic Acid to UFA and Stirring Velocity in the Tung Oil Epoxidation. Key Eng. Mater. 2021, 884, 117–124. [Google Scholar] [CrossRef]
- de Haro, J.C.; Izarra, I.; Rodríguez, J.F.; Pérez, Á.; Carmona, M. Modelling the epoxidation reaction of grape seed oil by peracetic acid. J. Clean. Prod. 2016, 138, 70–76. [Google Scholar] [CrossRef]
- Chen, J.; Beaufort, M.d.L.; Gyurik, L.; Dorresteijn, J.; Otte, M.; Gebbink, R.J.M.K. Highly efficient epoxidation of vegetable oils catalyzed by a manganese complex with hydrogen peroxide and acetic acid. Green Chem. 2019, 21, 2436–2447. [Google Scholar] [CrossRef]
- Budiyati, E.; Rochmadi, R.; Budiman, A.; Budhijanto, B. Studies on epoxidation of tung oil with hydrogen peroxide catalyzed by sulfuric acid. Bull. Chem. React. Eng. Catal. 2020, 15, 674–686. [Google Scholar] [CrossRef]
- Budiyati, E.; Sofyan, H.M.; Irsyad, N.; Salsyabila, A.; Musthofa, M. Mass transfer and reaction rate parameters for the in-situ epoxidation of tamanu oil. Chem. Pap. 2024, 78, 4131–4141. [Google Scholar] [CrossRef]
- Sawitri, D.R.; Mulyono, P.; Rochmadi; Hisyam, A.; Budiman, A. Kinetic investigation for in-situ epoxidation of unsaturated fatty acid based on the Pseudo-steady-state-hypothesis (PSSH). J. Oleo Sci. 2020, 69, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Raofuddin, D.N.A.; Azmi, I.S.; Jalil, M.J. Catalytic Epoxidation of Oleic Acid Derived from Waste Cooking Oil by In Situ Peracids. J. Polym. Environ. 2024, 32, 803–814. [Google Scholar] [CrossRef]
- Nwosu-Obieogu, K.; Grace, E.; Dzarma, G.W.; Aguele, F.O.; Chiemenem, L.I.; Gabriel, O.; Allen, M.; Ekeoma, N. Melon seed oil epoxidation: Kinetics and neuro-fuzzy evaluation. S. Afr. J. Chem. Eng. 2024, 47, 169–177. [Google Scholar] [CrossRef]
- Junaidi, J. The Ancillary Products ff Rubber (Hevea brasiliensis Muell. Arg.): Potential Resources to Enhance Sustainability. Agric. Socio-Econ. J. 2022, 22, 169. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).