1. Introduction
The data collation and analysis of analytical performance specific for two powerful label-free detection techniques that are attracting increased attention for many applications, namely surface plasmon resonance (SPR) and quartz crystal microbalance (QCM), have been reviewed many times in the literature (one of the first “ratings” can be found in ref. [
1]). These studies have typically compared technique-specific measures for a certain class of analytes, usually assuming that the instruments exploit the classical physical effects underlying these methods.
In methods based on the phenomenon of surface plasmon resonance, the information response is caused by the binding/localization of the analyte on the surface of the physical transducer or near it; changes in the optical characteristics caused by the presence of the analyte affect the excitation conditions or propagation of the plasmon–polariton excitation [
1,
2]. Thus, in the case of SPR, the information signal is a result of changes in the external environment located in the immediate vicinity of the physical transducer surface. Different realizations of SPR devices exploit various aspects of the effect, but it is the changes in the external environment that invariably generate the information signal for the sensor. More precisely, the response of an SPR sensor is determined by the intersection of the spaces occupied by the evanescent wave and the region in which the change in the absorption or refractive index occurs [
2]. In a rough approximation, the amount of non-absorbing analyte is proportional to the product of the change in the refractive index Δn and the thickness of the layer d in which these changes occur. A conceptually different approach uses the interference pattern generated by the superposition of propagating surface waves under SPR excitation conditions [
3,
4]. However, this approach to visualize, identify and count nano-objects has not yet found wide applications in analytical chemistry.
In resonance methods based on the concept of quartz microbalance, the situation is, in some sense, diametrically opposed. This method is based on the Sauerbrey theory, according to which the informative signal is formed as a result of an increase in the inertial mass of the physical transducer (PT) itself [
1,
5,
6]. For example, due to “rigidly fixed” adsorption on the PT surface (i.e., when the analyte is fixedly attached to the surface and moves in phase with it), the total mass of the oscillatory system increases, and accordingly, its resonant frequency decreases (compared to the case when the analyte is absent on the surface). Thus, in the classical Sauerbrey model, the effects of the external environment are completely absent, and the properties of the newly added mass (density, mechanical properties, etc.) are indistinguishable from the properties of the material of the oscillating plate (bulk-wave resonator). In this case, the source of the information signal is the characteristics of the physical transducer itself, without taking into account the external environment; the obvious differences between the material of the adsorbed layer and the metal of the electrode or the quartz crystal are not taken into account.
Of course, there have been numerous attempts to expand the basic concepts of the approaches discussed above, some of which have proven to be quite successful. The most promising, probably, is the development of the QCM-D technology, which makes it possible to identify the effects caused by the viscoelastic properties of the sensitive layer associated with the surface of the PT [
6,
7]. In this case, the limitations of the classical Sauerbrey theory were overcome and it was possible to take into account the fact that the properties of the organic coating of the sensitive layer differ from the properties of the materials from which both the electrodes and the bulk-wave resonator are made. However, the technical solution of the QCM-D technique involves a significant complication of the design of the electronic circuits and requires the resonator to operate in a cyclic mode (on-off), and the results cannot always be interpreted unambiguously. Therefore, traditional approaches based on the SPR and QCM techniques are still relevant, and the search for expanding the analytical capabilities of their classical implementation remains a trend in modern analytical science.
The aim of this work was to demonstrate how classical nanomaterials, represented by metallic nanoparticles stabilized by organic shells of various compositions, can contribute to the development of time-tested methods based on surface plasmon resonance effects and quartz crystal microbalances, in particular, to evaluate which of these methods, when routinely implemented, allows one to go beyond the classical description and establish a new concept of analytical approaches for the selective determination of the given gaseous analytes with high reliability.
2. Methodology for the Analytical Determination of an Analyte Based on Dynamic Processes Initiated by Adsorption in Sensor Architectures: Organically Stabilized Metal Nanoparticles as the Active Components of Chemical Sensors
Expanding the capabilities of the classical implementation of the SPR and QCM methods of analyte determining involves the inclusion of new mechanisms that are not provided for or have been leveled in the basic description of these approaches. To do this, it is necessary to first determine sensor parameters that affect their analytical information content.
In the case of surface plasmon resonance, the magnitude of the response is affected not only by changes in the absorption coefficient and refractive index (which are formed as a result of localization/adsorption of the analyte), but also by the distance from the PT surface at which this occurs. Due to the exponential decay of the evanescent wave intensity, a change in the initial thickness of the surface coating without changing its total mass leads to a change in the SPR response, since the overlap integral with the space occupied by the evanescent wave changes due to a variation in the coating structure. For example, the response of an SPR sensor decreases if the film “swells” without changing its composition, since after “expanding”, most of the film falls into the region where the intensity of the evanescent wave is lower. The manifestation of swelling in sensitive layers has been observed by many researchers, since it is inherent in many spatially organized structures under the influence of external factors, in particular, adsorption as an initiating factor [
8].
In the case of quartz microbalances, the resonance conditions are a consequence of the behavior of the oscillating inertial mass: if the mass of an object behaves as a single whole and all its parts oscillate in phase, then we are dealing with the Sauerbrey world, to which only a few corrections can be added that take into account the influence of the external environment on the process of oscillations of the physical transducer. The fact is that, unlike the spatially stationary PT SPR, the quartz resonator of bulk waves experiences complex spatial movements; in this case, the velocity and acceleration of its surface elements during movement can reach enormous values [
9]. Under these conditions of extreme accelerations, the lag/lead in the acceleration/deceleration of the inertial mass associated with the transducer surface is an inevitable phenomenon in the overwhelming majority of cases. The only question is which process predominates in this case: a change in the variable connection with the surface (slippage and similar effects of surface friction) or the dominant contribution is made by the viscoelastic characteristics of the material itself (volume deformation of the material, leading to a delay in the reaction of various fragments of the oscillating structure relative to the surface of the PT). Possible scenarios of these processes have been considered in the literature [
10,
11]. In particular, we showed that both effects can be observed in the experiment: slippage in the case of latex particles [
12] and viscoelastic damping of the PT by labile organic layers [
11].
Classical nanostructured materials based on metal nanoparticles (MNPs) stabilized by organic polymers offer unique opportunities for the implementation of new advanced approaches. Indeed, the metal core of MNPs is an object with a large inertial mass (what is important for QCM) and is capable of significantly affecting the optical characteristics of the medium in which they are embedded (what is important for SPR). On the other hand, organic coatings on the surface of MNPs are capable of playing the role of a nanoactuator, changing their geometric dimensions due to, for example, analyte sorption; this effect is especially pronounced when the stabilizing coating consists of high-molecular compounds and is capable of adopting various conformational states with a significant change in the area of the space it occupies. Polymer swelling and similar effects of responsive polymers (i.e., a change in structure under the influence of certain physical factors or environmental conditions, in particular, the binding of low-molecular compounds) is an inherent property of most macromolecules. In this sense, polymers such as branched polyethylenimine (BPEI), which is used in this work as a surface stabilizer for silver nanoparticles with a diameter of 60 nanometers, are no exception. Silver nanoparticles stabilized with a classic citrate shell consisting of low-molecular-weight citric acid residues were chosen as an alternative coating.
3. Experimental Section
Chemicals (acids (HCl, 37%, H2SO4 (37%), Roth), hydrogen peroxide (H2O2, 30%, Roth) and silver nanoparticles (the stock solution Sigma-Aldrich (St. Louis, MI, USA) 730817 Silver, Dispersion (60 nm); 60 nm Citrate NanoXact Silver; 60 nm BPEI NanoXact Silver, all with concentration of 0.02 mg/mL) were purchased from the above commercial suppliers and were used without further purification. All solutions were prepared according to the standard procedures using Milli-Q-purified water.
The SPR chips (20 × 20 × 1 mm
3 with 50 nm Au evaporated on Cr adhesive layer) were cleaned in a Piranha solution and nano-polished in 3% HAHP ({3 mL}HCl:{3 mL}H
2O
2:{94 mL}H
2O (
v/
v/
v) [
13]). After the treatment, the chips were washed in distilled water and dried in a stream of dry air. Droplets of 50 microliters of the stock suspension (0.02 mg/mL) were applied to the area of one of the chip halves. The samples were dried at room temperature in a Petri dish protected from light by aluminum foil. The droplet size of the nanoparticle suspension on the surface was about 50 mm
2 and varied slightly for different types of suspensions. However, given that the test beam size of the SPR spectrometer is about 15 mm
2 and shifts during scanning, the measurement result is an average value over an area comparable to the size of the dried droplet.
An SPR spectrometer “Biosuplar” (
www.biosuplar.de accessed on 15 June 2022) with angular scanning and a GaAs laser as the source of excitation (at a wavelength of 650 nm) was used. The SPR chips were fixed to a supporting glass prism (refractive index 1.51). For measurements, a scan (ca. 7°) mode was used. The measurements were performed in the flow mode with air as buffer gas. The 2-channel cell was formed by silicone rubber ~1 mm thick, covered with a PMMA holder mounted directly on the sensor chip surface. After a stable baseline value was established, the cell was injected by analyte vapors. After achieving equilibrium, the analyte was removed by purging with dry air.
For the QCM measurements, 7 microliter drops of the stock suspension were applied to the center of the metal (silver) electron on both sides of the QCM’s PT. After drying for 24 h, the procedure was repeated. The load measurement (i.e., the change in the quartz resonance frequency before and after the application and drying of the suspension) shows the close load values (1000 ± 300 Hz); the difference is due to the initial differences in the concentrations of the initial suspensions, and, in part, the uneven distribution of nanoparticles over the area of the quartz transducer (the sensitivity of the QCM has a Gaussian profile with a maximum in the center of the metal electrode [
9]). It should be emphasized that when analyzing the data, the mass of the polymer coating can be neglected, since the inertial mass of a silver nanoparticle with a diameter of 60 nanometers is about 6 × 10
8 Da (10.5 g/cm
3 × (4/3 × 3.1415 × (30 nm)
3 ~ 1.2 × 10
−15 g ~ 6 × 10
8 Da, based on the density of bulk silver and the hypothesis that the particles are perfectly spherical), which is 4 orders of magnitude greater than the molecular mass of the BPEI polymers used to stabilize the metal core (25 kDa, according to manufacturer’s specification). It is interesting to note that silver nanoparticles are approximately 2 times lighter than gold due to their lower density.
In this work, a home-made QCM-based array with a measurement step of 1 s and 1 Hz accuracy of 10 MHz quartz crystals (AT-cut, RK169) was used [
14]. The measuring procedure involved the following stages at room temperature: argon circulation until the transducer frequency is stabilized (±2 Hz); gas mixture circulation at a gas-carrier flow; circulation with argon until the QCM frequency returns to its initial value.
The following two types of liquids served as the test samples: vapors of distilled water (resistance 55 μS/cm) and ethyl alcohol (Chemsolute) were obtained by bubbling the solvent with a stream of purified air/argon at a carrier gas flow rate of about 100 mL/min.
4. Results and Discussion
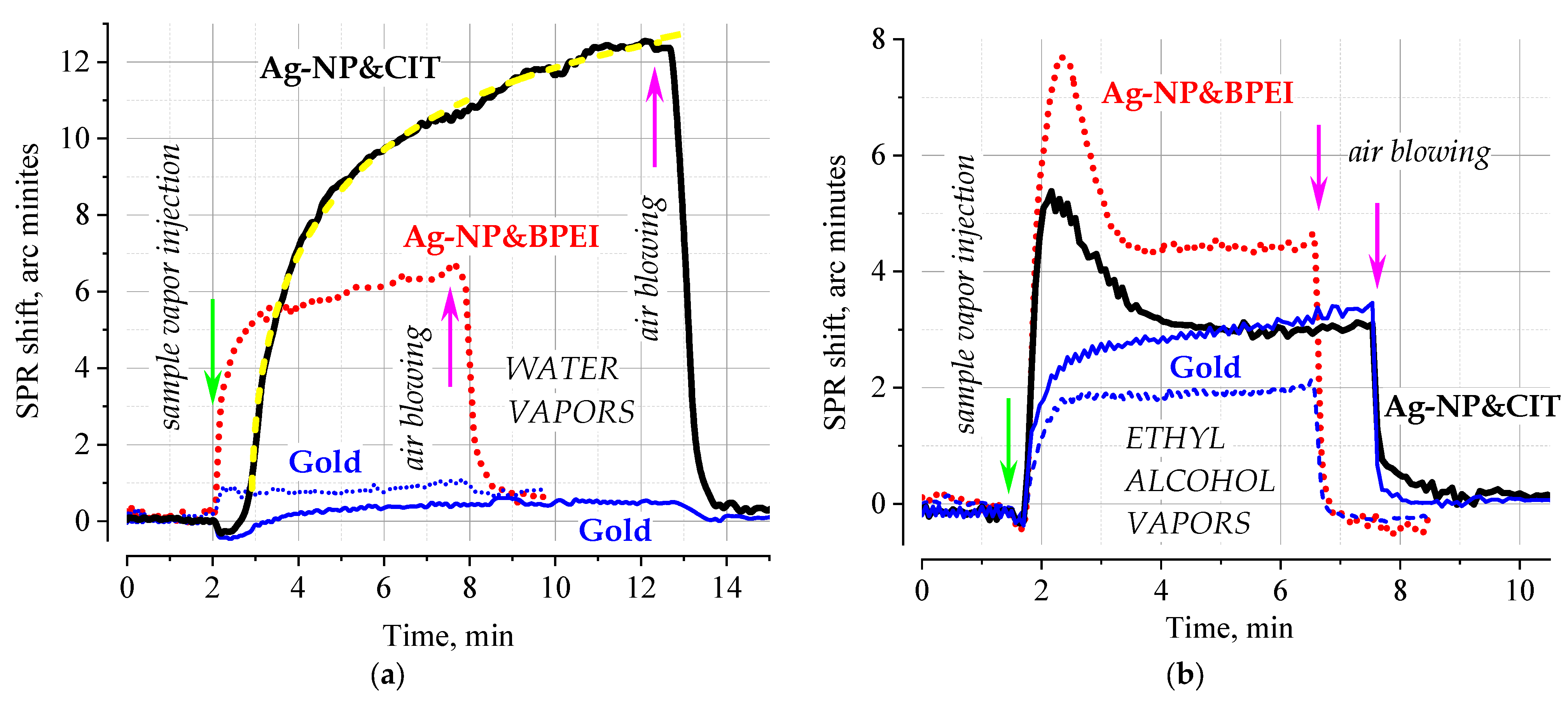
Figure 1 shows typical dependences of the SPR response to water and ethyl alcohol vapors for two coatings based on silver NPs stabilized with low-molecular-weight citric acid residues and a BPEI polymer. From the data presented in the figure, it follows that the response of water and ethyl alcohol to the surface of purified gold is about 1 and 2–3 arc minutes, respectively. The analysis of the kinetics of monotonic dependences (i.e., with the exception of dependences for ethyl alcohol shown in the
Figure 1b) indicates that the limiting stage of adsorption is the diffusion process. As an illustration in
Figure 1a, the best approximation is shown by the dashed line in yellow with a stretched exponential function with a β value of 0.5 [
15]; the reaction is governed by a square root time dependence for both modified surfaces and pure gold. For all the studied cases, the adsorption process is reversible; after the analyte is removed from the headspace, the baseline returns to its original value. This indicates the physical nature of the adsorption process, which is not accompanied by the formation of new chemical compounds.
The analysis of experimental data confirms that nanoparticle deposition introduces some chemical functionality into the interface; the response to the analyte depends on the type of coating and the analyte. In the case of Ag-NP&CIT, the response to water vapor increased more than 20 times. Somewhat surprising are the practically comparable values of the response of the Ag-NP&CIT coating and pure gold to ethyl alcohol vapor. However, as in the case of Ag-NP&BPEI, the type of response indicates that in these cases, the structure of the sensitive layer changes due to the adsorption of the analyte (rapid increase in the response), caused by swelling with increasing coating thickness (drop in the response) and subsequent stabilization of the new configuration (reaching a steady-state response value). In line with model calculations, for such an effect to manifest itself in the SPR method, a small (sub-nanometer range) shift of 1–2 layers of nanoparticles in the direction perpendicular to the substrate is sufficient. Indeed, the diameter of silver nanoparticles is about 60 nm, and the main energy of the evanescent wave is concentrated in the region of tens of nanometers from the surface; a small change in the position of the 60 nm nanoparticles in this region is enough to compensate for the effect caused by the adsorption of the low-molecular weight analyte. Such changes are observed only during the first exposure to ethanol vapor; subsequent cycles demonstrate a typical monotonic dependence characteristic of the diffusion-limited adsorption mode with similar values of response. Such behavior is typical for organic materials [
16], and, as we can now conclude, for nanostructured objects in an organic shell also: under the action of the solvent, the spatial structure passes into a thermodynamically equilibrium state, relieving the deformation stresses that arose during application under nonequilibrium conditions.
Summarizing the results obtained by the SPR method, it can be stated that the use of organic modified metal nanoparticles leads to an increase in the selective response due to the chemical nature of the organic layer. At the same time, the general picture of the adsorption process is typical for most organic materials that do not contain embedded nanostructures. This allows us to suggest that the use of functional nanostructures in conventional SPR format does not provide any unique opportunities for the analytical determination of gaseous analytes.
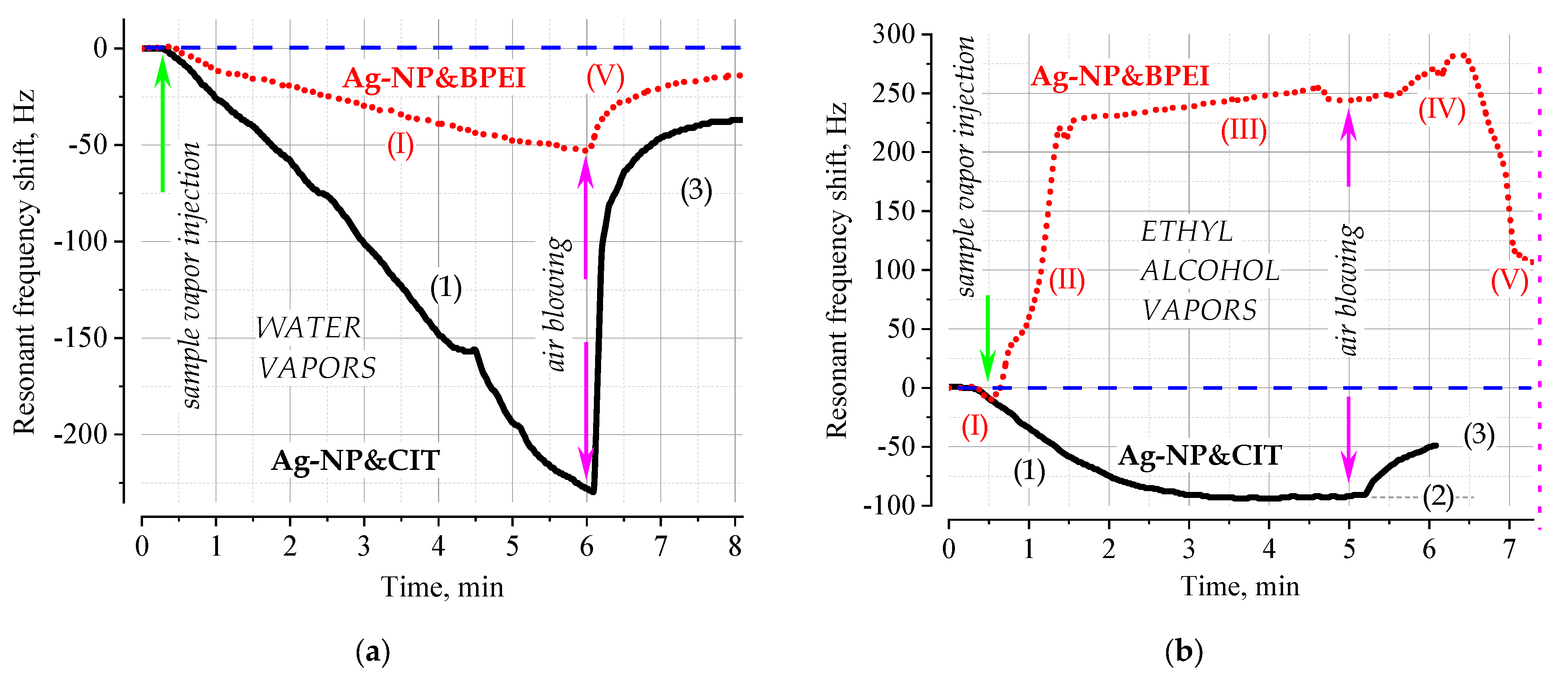
A typical sorption pattern is also observed for QCM transducers with an injection of saturated water vapor (
Figure 2a). The sorption process is characterized by a fairly long process of monotonic decrease in the resonance frequency of the QCM’s PT in the gas phase with a “quasi-linear” dependence of the response versus time; such a dependence is typical for the case when the analyte not only binds to the surface, but also penetrates deeper into the structure of the sensitive layer [
17]. Considering the fact that the surface roughness of the conventional commercially-available quartz crystal oscillators is large (micron range), the most plausible process seems to be formation of multilayer nanoparticle structures in the valleys of the surface structure. In recessed areas, the suspension of nanoparticles collects into separate clusters, which limits its movement by spatial walls. This distinguishes the surface organization of the layer in the case of SPR’s PTs, where the roughness of the gold film is in the nanometer range. Despite the differences in the local organization of the sensitive layer on the smooth surface of gold and rough silver, the adsorption/sorption process is reversible; moreover, for water vapor ones, it occurs in accordance with the classical Sauerbrey model. In general, the behavior of the sensitive layers in both methods under the flow of water vapor is similar: (1) both methods reliably register the presence of the analyte; (2) the selectivity of adsorption is determined by the properties of the coating, both methods demonstrate a greater response with a citric acid coating capable of effectively forming hydrogen bonds; (3) the adsorption process is reversible, indicating the dominance of the physical sorption mechanism; and (4) the signal-to-noise ratio is somewhat better in the case of QCM transducers, which is typical for gaseous analytes.
The picture of surface processes changes dramatically upon the injection of ethyl alcohol vapors (
Figure 2b). While the response of the Ag-NP&CIT-based coating is typical for reversible surface adsorption in the Sauerbrey world, the response of the PT modified by Ag-NP&BPEI demonstrates typical anti-Sauerbrey behavior: the resonance frequency increases, non-monotonic response behavior is observed, and analyte desorption leads to reverse relaxation of the informative sensory signal only after some time. In this case, a large increase in the resonance frequency is observed, which indicates significant changes at the interface, which lead to a violation of the main provisions of the Sauerbrey theory [
6,
11,
12].
Considering that in the case of QCM’s PT nanoparticles are concentrated in the surface depressions, it can be reasonably assumed that a stochastic array of cup-shaped voids filled with a suspension of nanoparticles capable of passing into a gel state under the action of analyte vapors is formed at the interface. The synchronicity of the motion of the massive metal cores of the nanoparticles and the surface of the quartz transducer under the action of alternating accelerations of the oscillating crystal is ensured by the adhesive properties of the polymer coating of the nanoparticles. Considering the high adhesive capacity of BPEI [
18] and the presence of steric hindrances perpendicular to the direction of motion of the quartz plate during oscillations, the “continuity” of the movements of the entire structure is preserved both in the “dry” state and in the presence of water vapor. This is due to the fact that BPEI is soluble only in “hot” or “cold water at a low pH value” [
19]. At the same time, BPEI dissolves well in ethanol, and it is ethanol that can have a plasticizing effect and even increase the foaming efficiency of the BPEI polymer mass [
20]. Indeed, a rheology study shows that an ethanol-based suspension of BPEI has a lower viscosity than a water-based ones [
21]. It is also worth noting the unique feature of the linear regions of poly(ethyleneimine) ([-CH
2CH
2NH-]
x) with their conformational- and configurational-adjustable properties: the hydrogen atom and the lone pair on the same nitrogen atom easily exchange positions [
19]. Such nitrogen inversion tends to increase the configurational entropy, enhance solubility, and can cause rapid changes in the conformation of the polymer backbone. The probability of rapid nitrogen inversion is determined by the presence of nearby NH groups and depends on the possibility of intramolecular interactions (N–H· · ·N) controlled by a given environment, in particular analyte molecules. While ethanol has an oxygen atom with a lone pair, it can form a hydrogen bond with the polymer nitrogen, creating steric hindrance via a hydrophilic methyl group to the reverse process. In the case of water molecules, this process may be reversible due to the absence of steric hindrance.
All this taken together allows us to consider that the most probable mechanism of the processes leading to the anti-Sauerbrey behavior of Ag-NP&BPEI coating is associated with the disruption of the strong adhesive contact between the NPs and the surface and neighboring nanoparticles (“rattle”). In this case, conditions are created for the asynchronous movement of gel-like aggregates of nanoparticles (within the surface depressions) and the surface of the quartz resonator. Thus, sorption-initiated changes in the adhesion and viscoelastic properties of the surface architecture of nanoparticles on the surface of the QCM’s PTs led to the manifestation of anti-Sauerbrey behavior.
5. Concluding Remarks
The differences in the adsorption processes characteristic of the classical and “stimulus-responsive” (anti- Sauerbrey) behavior are that instead of the typical processes of surface binding (1) limited by the adsorption capacity of the surface with the maximum filling level (2) and subsequent desorption, (3) a much more complex process takes place (
Figure 2). The main feature of this complex behavior is that the usual surface binding (I) causes a change in the macrostructure of the coating (II), for which the limiting response of QCM’s PT is due not only to the inertial mass of the attached analyte, but also to the changed viscoelastic/friction properties of the coating (III) (adsorption induces swelling [
8,
22]). Upon desorption of the analyte, such a structure first becomes even more labile due to the loss (IV) of a part of its bonds fixed by the analyte, and only after a certain critical number of analyte molecules have been removed, the structure begins to relax to its thermodynamically stable state characteristic of the medium without the analyte (V). These dynamic processes initiated by adsorption on responsive polymer nanoactuators and amplified by the presence of “heavy” metal nanoparticles with high inert mass allows the use of a fundamentally different approach to detecting analytes than the loading-based traditional one. This scenario offers broad prospects for the development of a new class of highly selective sensors, where not only the magnitude of the response, but also its sign will allow for the desired analyte to be uniquely identified from a number of similar ones and quantitatively characterized.
The results of this work allow us to breathe new life into time-tested experimental techniques. Unfortunately, despite significant advances in the development of analytical methods based on both SPR and QCM, they have not yet found wide application as reliable devices for medicine and ecology. This is largely due to the fact that hopes that the development of electronics and computer technology will allow us to overcome the existing problems have not been justified. Despite significant progress in the application of biosensors, significant efforts are still needed to make laboratory prototypes widely available [
23]. The existing problems that hinder the widespread use of these promising physical approaches are more fundamental in nature. The conceptual foundations of new types of sensitive elements demonstrated in the report once again emphasize the fact that more and more attention should be paid to fundamental scientific problems, without solving which no technical solutions can become full-fledged devices. We hope that the methodological principles of selective analytical determination of an analyte demonstrated in this work, based on the effects of spatial dynamic relaxation of sensitive architectures, have made it possible to highlight some promising ways of creating actually feasible devices for solving analytical problems facing our technological civilization.