Advances, Benefits, and Challenges of Wearable Sensors for Healthcare and Stress Management: A Focus on Hemodynamic Parameters and Cortisol Measurement †
Abstract
1. Introduction
2. Continuous Noninvasive Blood Pressure (cNIBP)—Hemodynamics
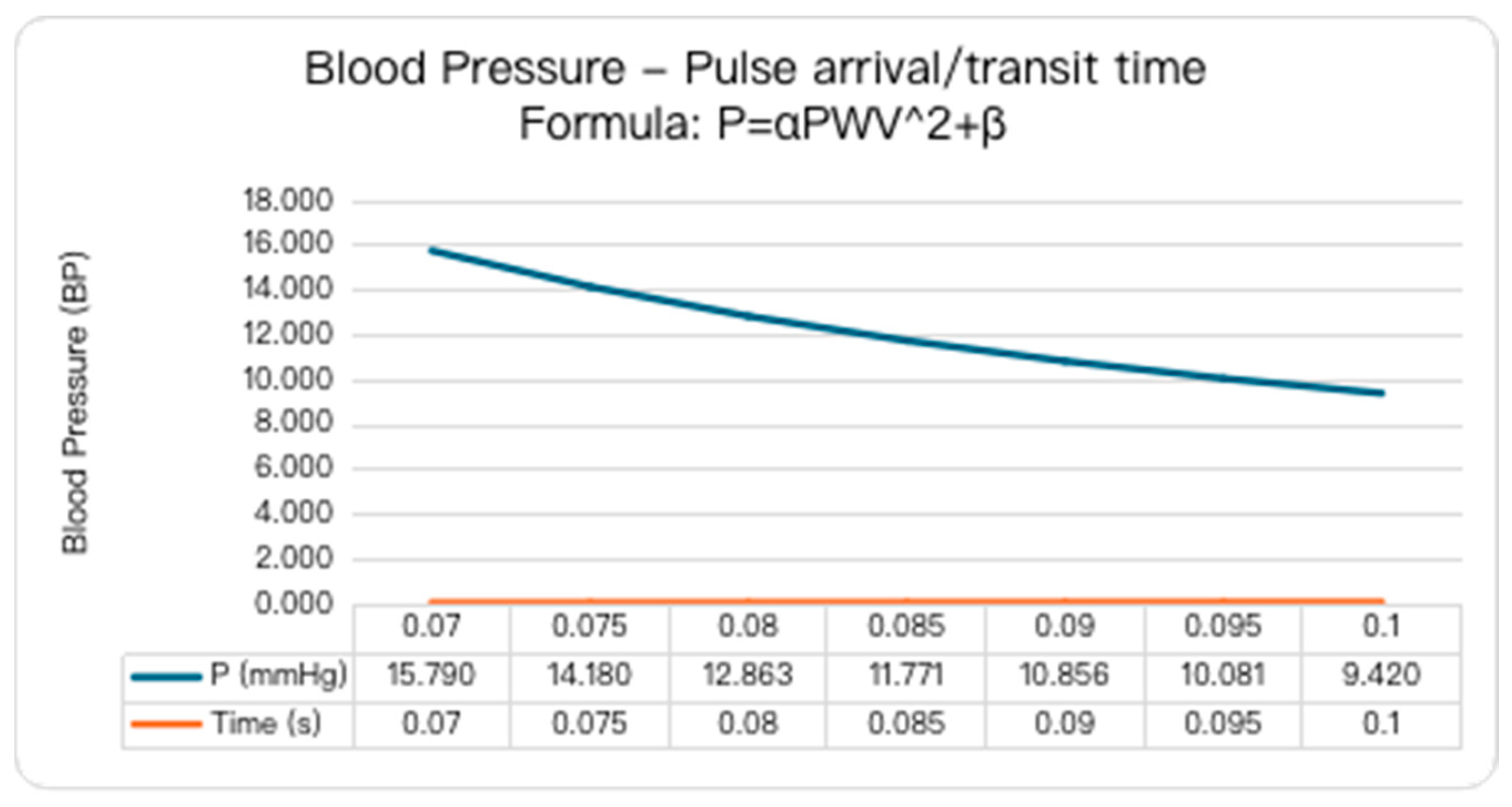
2.1. Blood Pressure Estimation
2.1.1. Photoplethysmography (PPG)
2.1.2. Impedance Plethysmography (IPG)
2.1.3. Ultrasound Imaging (UI)
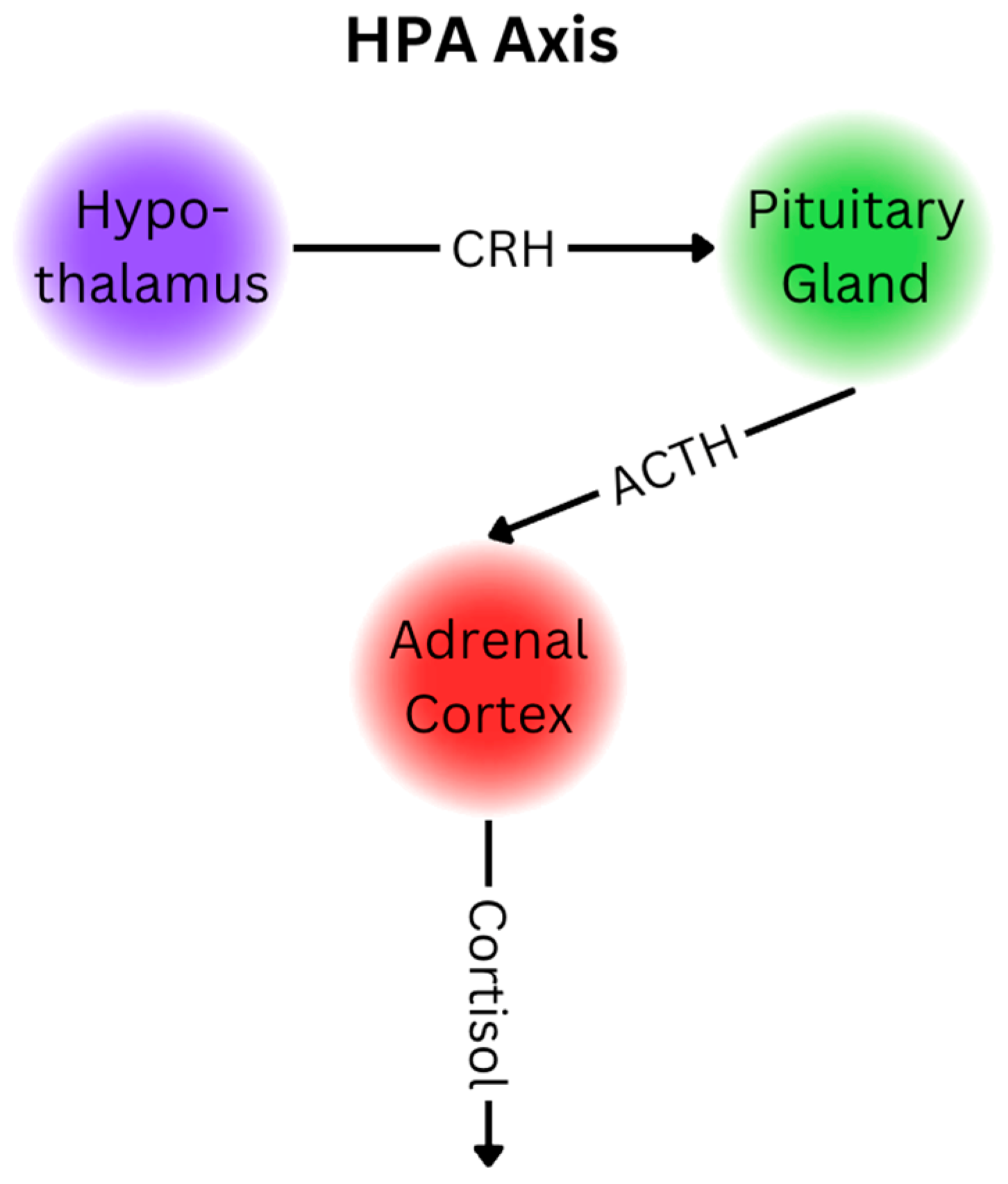
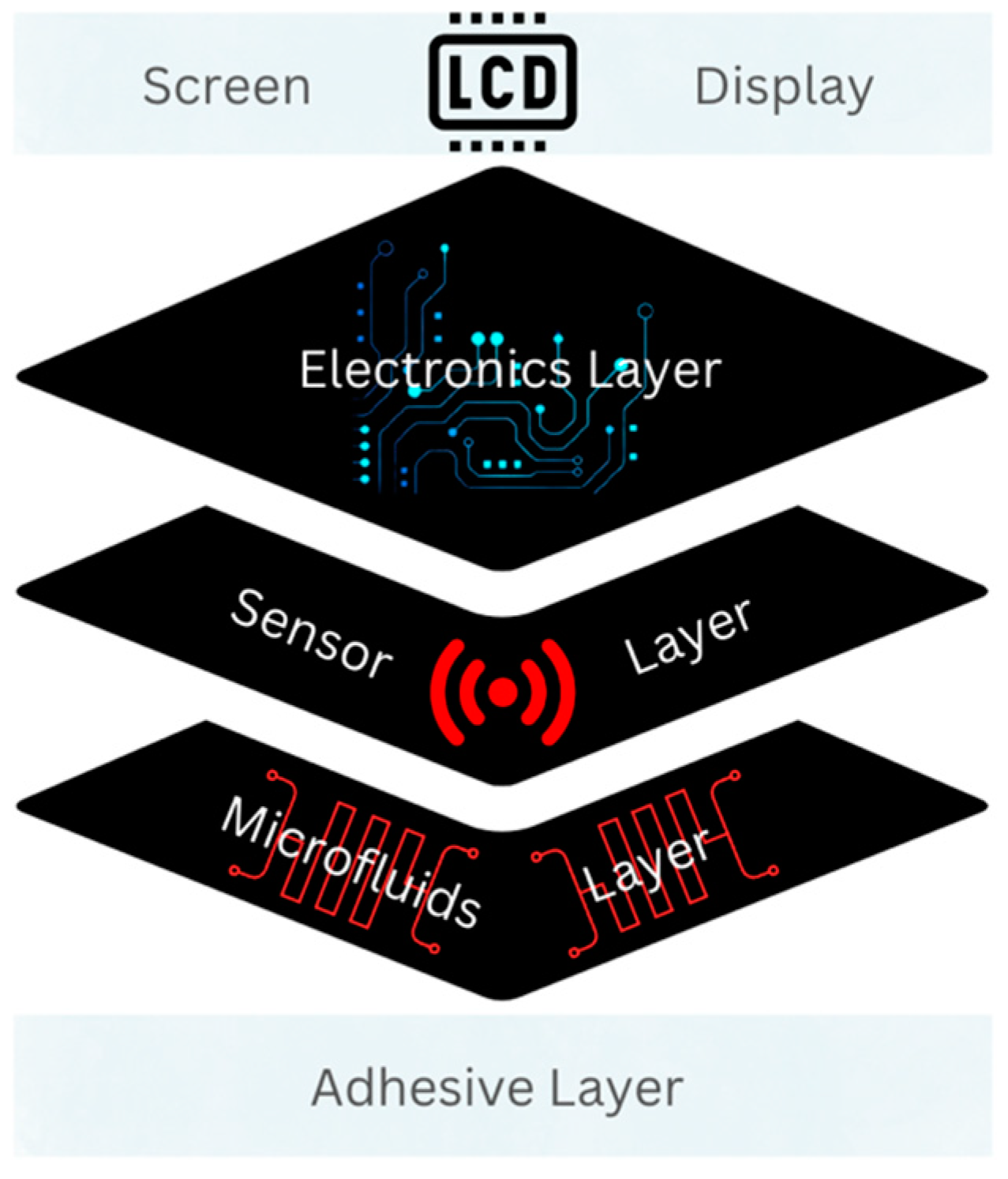
3. Cortisol
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Choi, J.; Hourlier-Fargette, A.; Xue, Y.; Chung, H.U.; Lee, J.Y.; Wang, X.; Xie, Z.; Kang, D.; Wang, H.; et al. Relation between blood pressure and pulse wave velocity for human arteries. Proc. Natl. Acad. Sci. USA 2018, 115, 11144–11149. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Hill, L.K.; Williams, D.P.; Thayer, J.F. Estimating cardiac output from blood pressure and heart rate: The liljestrand & zander formula. Biomed. Sci. Instrum. 2015, 51, 85–90. [Google Scholar]
- Kim, H.-L.; Kim, S.-H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Koike, A.; Kubota, H.; Wu, L.; Nishi, I.; Sato, A.; Aonuma, K.; Kawakami, Y.; Ieda, M. Pulse wave transit time during exercise testing reflects the severity of heart disease in cardiac patients. Drug Discov. Ther. 2020, 14, 21–26. [Google Scholar] [CrossRef]
- Liao, C.-F.; Cheng, H.-M.; Sung, S.-H.; Yu, W.-C.; Chen, C.-H. Determinants of pressure wave reflection: Characterization by the transit time-independent reflected wave amplitude. J. Hum. Hypertens. 2011, 25, 665–671. [Google Scholar] [CrossRef]
- Lokharan, M.; Lokesh Kumar, K.C.; Harish Kumar, V.; Kayalvizhi, N.; Aryalekshmi, R. Measurement of Pulse Transit Time (PTT) Using Photoplethysmography. In Proceedings of the 16th International Conference on Biomedical Engineering; Singapore, 4–7 December 2016, Goh, J., Lim, C.T., Leo, H.L., Eds.; Springer: Singapore, 2017; pp. 130–134. [Google Scholar]
- Kumar, P.V.G.; Deshpande, S.; Nagendra, H.R. Traditional practices and recent advances in Nadi Pariksha: A comprehensive review. J. Ayurveda Integr. Med. 2019, 10, 308–315. [Google Scholar] [CrossRef]
- Karimpour, P.; May, J.M.; Kyriacou, P.A. Photoplethysmography for the Assessment of Arterial Stiffness. Sensors 2023, 23, 9882. [Google Scholar] [CrossRef] [PubMed]
- Monte-Moreno, E. Non-invasive estimate of blood glucose and blood pressure from a photoplethysmograph by means of machine learning techniques. Artif. Intell. Med. 2011, 53, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Liu, H.; Lin, W.-H.; Zheng, D.; Chen, F. Filtering-induced changes of pulse transmit time across different ages: A neglected concern in photoplethysmography-based cuffless blood pressure measurement. Front. Physiol. 2023, 14, 1172150. [Google Scholar] [CrossRef]
- Myint, C.; Lim, K.H.; Wong, K.I.; Gopalai, A.A.; Oo, M.Z. Blood Pressure measurement from Photo-Plethysmography to Pulse Transit Time. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Miri, Malaysia, 8–10 December 2014; pp. 496–501. [Google Scholar]
- Ghosh, S.; Chattopadhyay, B.P.; Roy, R.M.; Mukherjee, J.; Mahadevappa, M. Non-invasive cuffless blood pressure and heart rate monitoring using impedance cardiography. Intell. Med. 2022, 2, 199–208. [Google Scholar] [CrossRef]
- Wang, T.-W.; Syu, J.-Y.; Chu, H.-W.; Sung, Y.-L.; Chou, L.; Escott, E.; Escott, O.; Lin, T.-T.; Lin, S.-F. Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement. Biosensors 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.H.; Jafari, R.; Chung, W.-Y. Noninvasive Cuffless Blood Pressure Estimation Using Pulse Transit Time and Impedance Plethysmography. IEEE Trans. Biomed. Eng. 2019, 66, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoo, H.-J. Simultaneous Electrical Bio-Impedance Plethysmography at Different Body Parts: Continuous and Non-Invasive Monitoring of Pulse Wave Velocity. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Vappou, J.; Luo, J.; Konofagou, E.E. Pulse Wave Imaging for Noninvasive and Quantitative Measurement of Arterial Stiffness In Vivo. Am. J. Hypertens. 2010, 23, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Belen, A. Estimation of Blood Pressure by Image-Free, Wearable Ultrasound. Artery Res. 2024, 30, 3. [Google Scholar] [CrossRef]
- Kenny, J.-É.S.; Munding, C.E.; Eibl, A.M.; Eibl, J.K. Wearable ultrasound and provocative hemodynamics: A view of the future. Crit. Care 2022, 26, 329. [Google Scholar] [CrossRef]
- Zakrzewski, A.; Anthony, B. Non-Invasive Blood Pressure Estimation Using Ultrasound and Simple Finite Element Models. IEEE Trans. Biomed. Eng. 2017, 65, 2011–2022. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X. See how your body works in real time—Wearable ultrasound is on its way. Nature 2024, 630, 817–819. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Navani, N.K.; Li, Y. Nucleic acid aptamers and enzymes as sensors. Curr. Opin. Chem. Biol. 2006, 10, 272–281. [Google Scholar] [CrossRef]
- Debiais, M.; Lelievre, A.; Smietana, M.; Müller, S. Splitting aptamers and nucleic acid enzymes for the development of advanced biosensors. Nucleic Acids Res. 2020, 48, 3400–3422. [Google Scholar] [CrossRef] [PubMed]
- Wassel, E.; Umeh, C.A.; Giberson, C.; Anand, S.K.; Nguyen, A.; Porter, H.; Choday, P.; Kaur, H.; Kundu, A.; Penaherrera, J. Elevated Adrenocorticotropic Hormone, Hypercortisolism, and Marked Hypernatremia. Cureus 2021, 13, e19714. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Taskasaplidis, G.; Fotiadis, D.A.; Bamidis, P.D. Review of Stress Detection Methods Using Wearable Sensors. IEEE Access 2024, 12, 38219–38246. [Google Scholar] [CrossRef]
- Wu, C.-H.; Ma, H.J.H.; Baessler, P.; Balanay, R.K.; Ray, T.R. Skin-interfaced microfluidic systems with spatially engineered 3D fluidics for sweat capture and analysis. Sci. Adv. 2023, 9, eadg4272. [Google Scholar] [CrossRef] [PubMed]


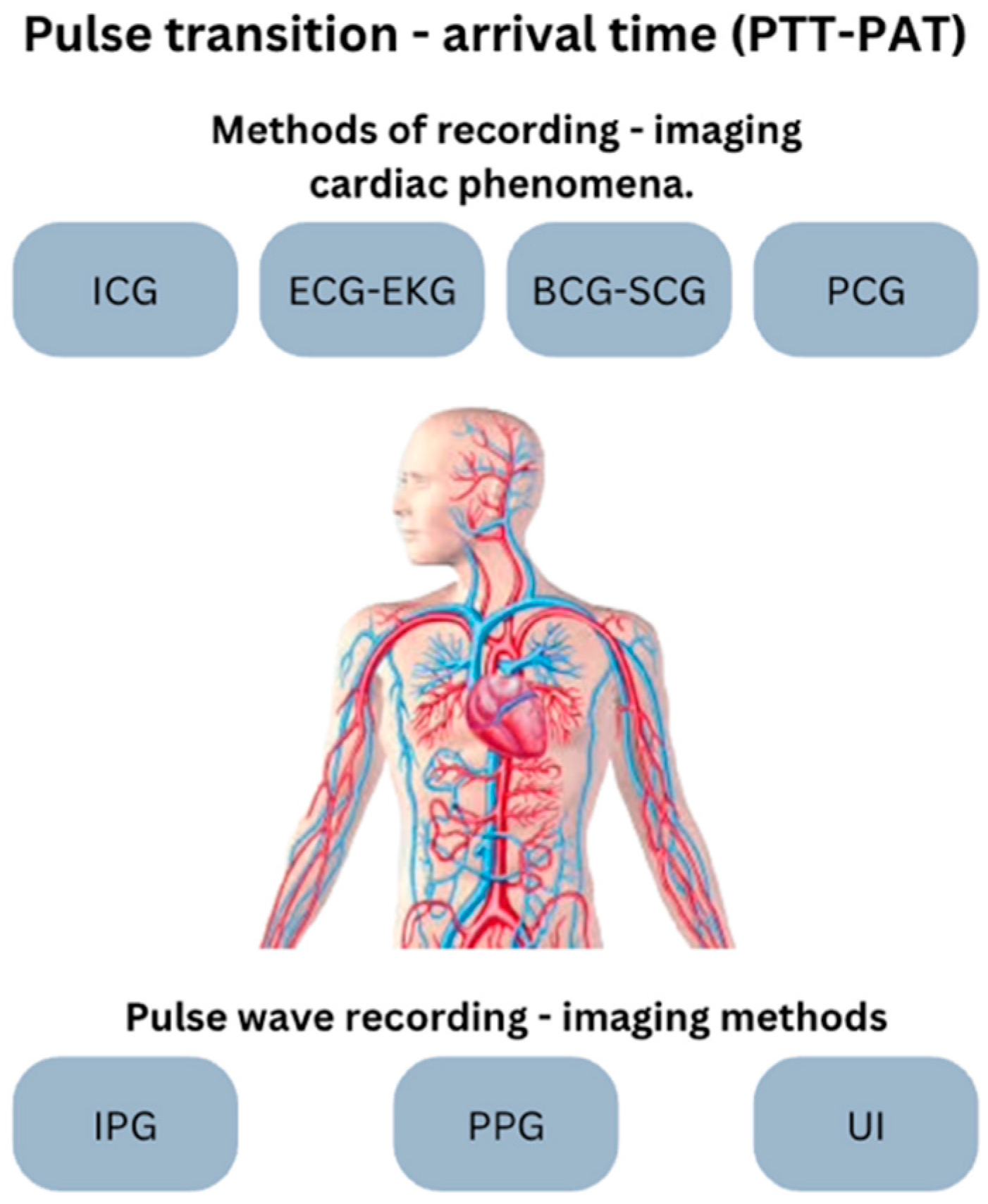
| Abbr. | Method | Means | Activity/Imaging | Measurement |
|---|---|---|---|---|
| BCG -SCG | Ballistocardiography Seismocardiography | Accelerator | Heart and aorta vibration (mechanical) | Heartbeat, vibrations of the heart and aorta |
| ECG-EKG | Electrocardiography Electrocardiography | Electrodes | Heart electric | R-R intervals, heartbeat |
| IPG | Impedance Plethesmography | Electrodes, electric power source | Heart mechanical | Heart pulse wave, arterial blood volume changes |
| PCG | Phonocardiography | Sound recorder | Heart sound | Heartbeat, sound and murmur recording |
| PPG | Photoplethysmography | Photodiode (led) | Blood flow | Heart pulse wave, blood flow and volume changes |
| UI | Ultrasound Imaging | Acoustic waves | Arterial, vein | Heart pulse wave, scattering of the blood cell particles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskasaplidis, G.V.; Liogas, K.A.; Korsunsky, A.M.; Fotiadis, D.A.; Bamidis, P.D. Advances, Benefits, and Challenges of Wearable Sensors for Healthcare and Stress Management: A Focus on Hemodynamic Parameters and Cortisol Measurement. Eng. Proc. 2024, 82, 42. https://doi.org/10.3390/ecsa-11-20492
Taskasaplidis GV, Liogas KA, Korsunsky AM, Fotiadis DA, Bamidis PD. Advances, Benefits, and Challenges of Wearable Sensors for Healthcare and Stress Management: A Focus on Hemodynamic Parameters and Cortisol Measurement. Engineering Proceedings. 2024; 82(1):42. https://doi.org/10.3390/ecsa-11-20492
Chicago/Turabian StyleTaskasaplidis, Georgios V., Konstantinos A. Liogas, Alexander M. Korsunsky, Dimitris A. Fotiadis, and Panagiotis D. Bamidis. 2024. "Advances, Benefits, and Challenges of Wearable Sensors for Healthcare and Stress Management: A Focus on Hemodynamic Parameters and Cortisol Measurement" Engineering Proceedings 82, no. 1: 42. https://doi.org/10.3390/ecsa-11-20492
APA StyleTaskasaplidis, G. V., Liogas, K. A., Korsunsky, A. M., Fotiadis, D. A., & Bamidis, P. D. (2024). Advances, Benefits, and Challenges of Wearable Sensors for Healthcare and Stress Management: A Focus on Hemodynamic Parameters and Cortisol Measurement. Engineering Proceedings, 82(1), 42. https://doi.org/10.3390/ecsa-11-20492










