Detection of Disease-Specific Volatile Organic Compounds Using Infrared Spectroscopy †
Abstract
:1. Introduction
2. Detection of Biomarkers Using Infrared Spectroscopy
3. Disease-Specific Biomarkers
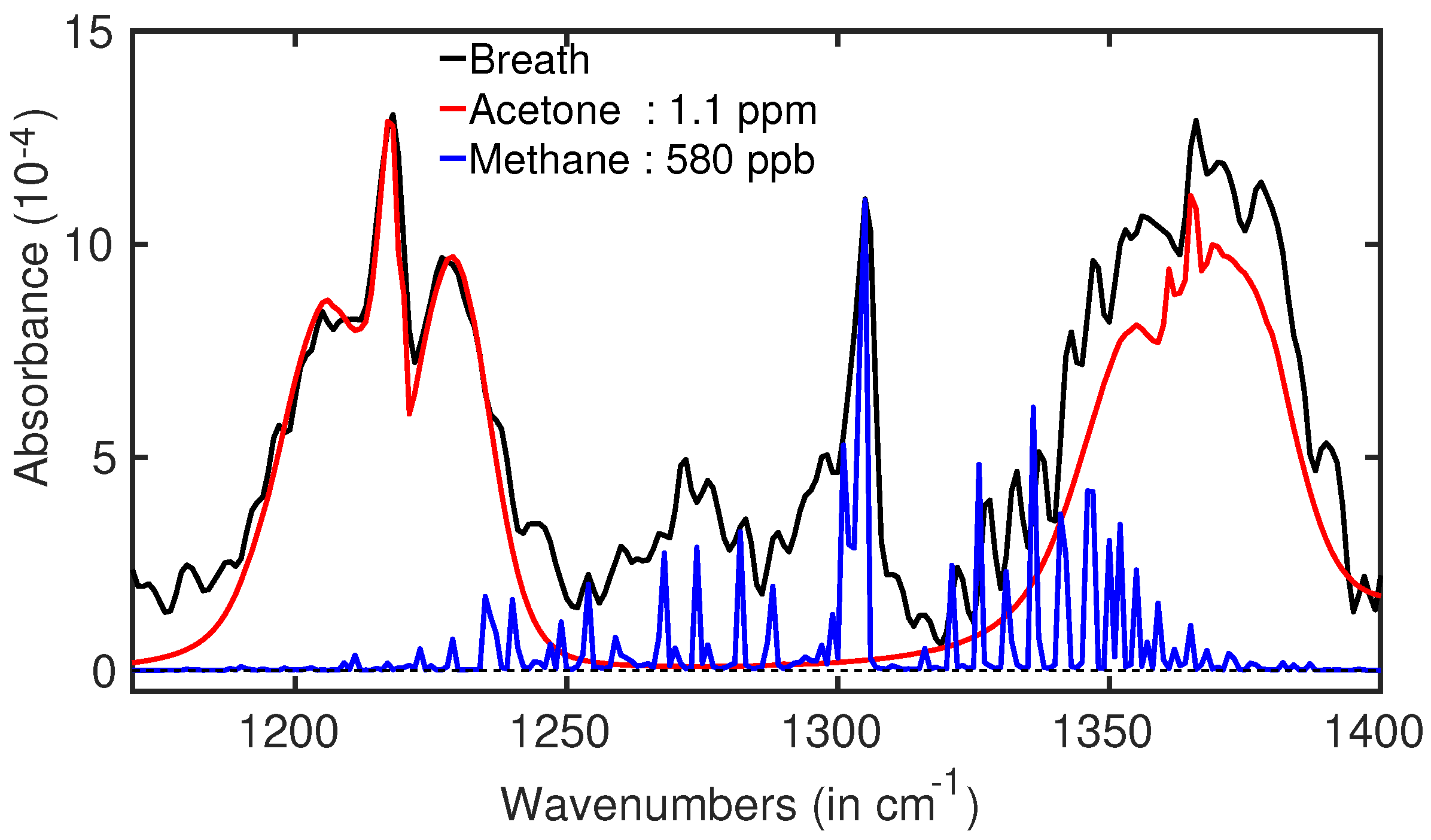
3.1. Diabetes
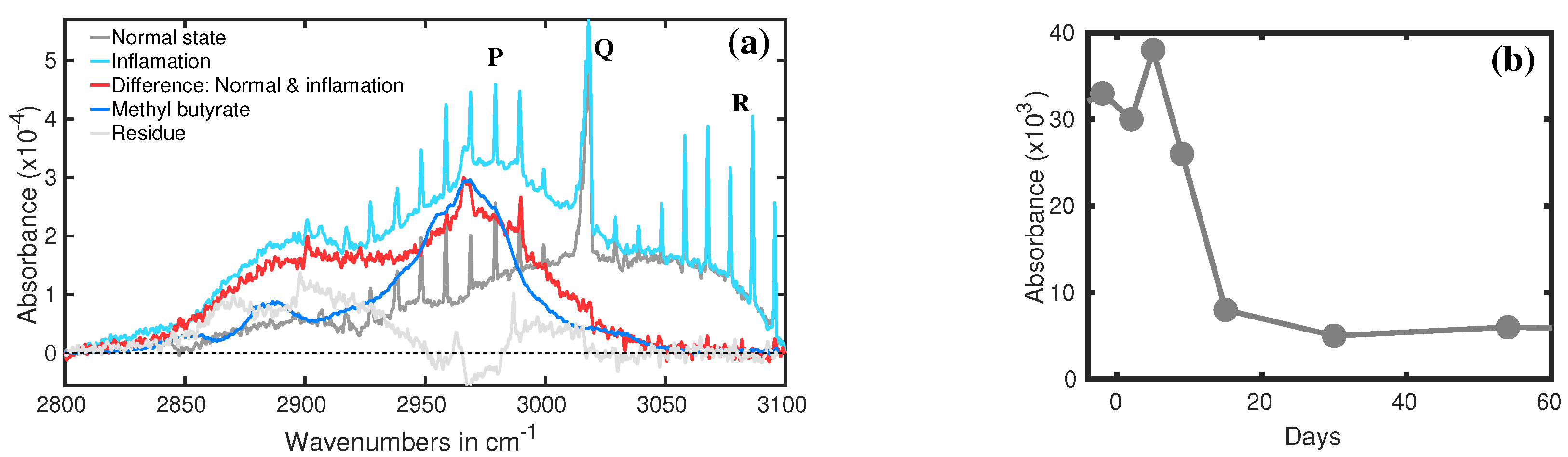
3.2. Antibiotic Treatment
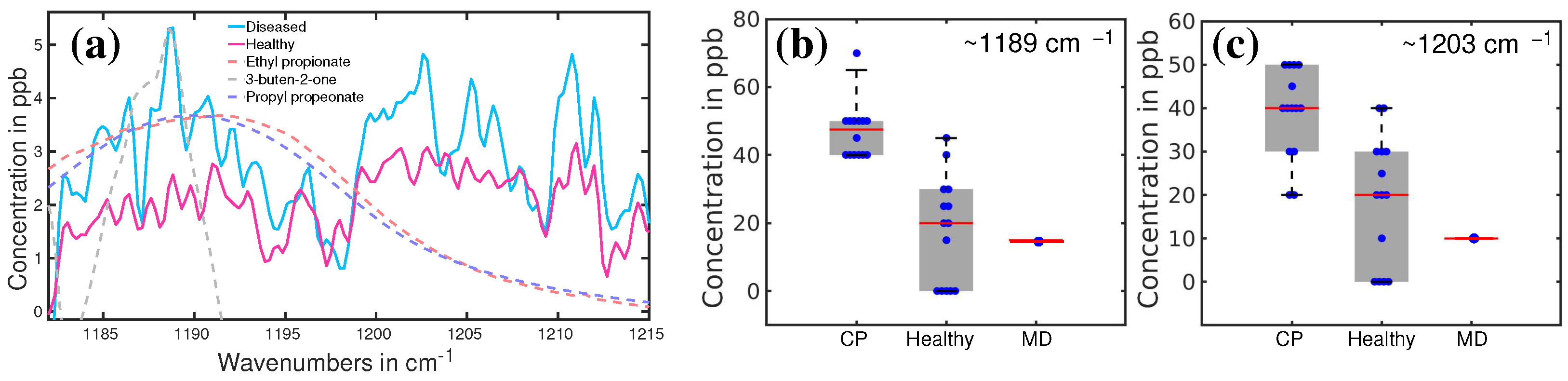
3.3. Cerebral Palsy
3.4. Prostate Cancer
4. Conclusions
Institutional Review Board Statement
Informed Consent Statement
References
- Metzler, D.E. Biochemistry: The Chemical Reactions of Living Cells; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonski, A. Human beings as islands of stability: Monitoring body states using breath profiles. Sci. Rep. 2019, 9, 16167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apolonski, A.; Roy, S.; Lampe, R.; Maiti, K.S. Molecular identification of bio-fluids in gas phase using infrared spectroscopy. Appl. Opt. 2020, 59, E36–E41. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.D.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lamote, K.; Van Cleemput, J.; Nackaerts, K.; Vandermeersch, L.; Van Langenhove, H.; van Meerbeeck, J.P. Breath analysis by gas chromatography-mass spectrometry can be used to screen for pleural mesothelioma. Eur. Respir. J. 2016, 48, OA499. [Google Scholar] [CrossRef]
- Apolonski, A.; Roy, S.; Lampe, R.; Maiti, K.S. Application of Vibrational Spectroscopy in Biology and Medicine. Breath Analysis. Proceedings 2019, 27, 26. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.C.; Rich, P.; Foreman, L.; Smith, J.; Yu, M.S.; Tanna, A.; Dibbur, V.; Unwin, R.; Tam, F.W.K. Label Free Detection of Sensitive Mid-Infrared Biomarkers of Glomerulonephritis in Urine Using Fourier Transform Infrared Spectroscopy. Sci. Rep. 2017, 7, 4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, K.S. Vibrational spectroscopy of Methyl benzoate. Phys. Chem. Chem. Phys. 2015, 17, 19735–19744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, K.S. Broadband two dimensional infrared spectroscopy of cyclic amide 2-Pyrrolidinone. Phys. Chem. Chem. Phys. 2015, 17, 24998–25003. [Google Scholar] [CrossRef] [PubMed]
- Apolonski, A.; Maiti, K.S. Towards a standard operating procedure for revealing hidden volatile organic compounds in breath: The Fourier-transform IR spectroscopy case. Appl. Opt. 2021, 60, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Gelin, M.F.; Blokhin, A.P.; Ostrozhenkova, E.; Apolonski, A.; Maiti, K.S. Theory helps experiment to reveal VOCs in human breath. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2021, 258, 119785. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonski, A. Sensitive spectroscopic breath analysis by water condensation. J. Breath Res. 2018, 12, 046003. [Google Scholar] [CrossRef] [PubMed]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Apolonski, A. Monitoring the Reaction of the Body State to Antibiotic Treatment against Helicobacter pylori via Infrared Spectroscopy: A Case Study. Molecules 2021, 26, 3474. [Google Scholar] [CrossRef] [PubMed]
- Stotz, S.; von Zawadzky, R.M. Therapie der infantilen Cerebralparese: Das Münchner Tageskonzept (Pflaum Physiotherapie); Richard Pflaum Verlag GmbH & Co. KG: Munich, Germany, 2000. [Google Scholar]
- Alpay Savasan, Z.; Yilmaz, A.; Ugur, Z.; Aydas, B.; Bahado-Singh, R.O.; Graham, S.F. Metabolomic Profiling of Cerebral Palsy Brain Tissue Reveals Novel Central Biomarkers and Biochemical Pathways Associated with the Disease: A Pilot Study. Metabolites 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, K.S.; Roy, S.; Lampe, R.; Apolonski, A. Breath indeed carries significant information about a disease. Potential biomarkers of cerebral palsy. J. Biophotonics 2020, 13, e202000125. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Fill, E.; Strittmatter, F.; Volz, Y.; Sroka, R.; Apolonski, A. Accurate diagnosis of prostate cancer via infrared spectroscopy of breath. In Proceedings of the European Conferences on Biomedical Optics 2021 (ECBO), Munich, Germany, 20–24 June 2021; p. ETu1A.3. [Google Scholar]
- Maiti, K.S.; Fill, E.; Strittmatter, F.; Volz, Y.; Sroka, R.; Apolonski, A. Towards reliable diagnostics of prostate cancer via breath. Sci. Rep. 2021, 11, 18381. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, K.S.; Roy, S.; Lampe, R.; Apolonski, A. Detection of Disease-Specific Volatile Organic Compounds Using Infrared Spectroscopy. Eng. Proc. 2021, 8, 15. https://doi.org/10.3390/engproc2021008015
Maiti KS, Roy S, Lampe R, Apolonski A. Detection of Disease-Specific Volatile Organic Compounds Using Infrared Spectroscopy. Engineering Proceedings. 2021; 8(1):15. https://doi.org/10.3390/engproc2021008015
Chicago/Turabian StyleMaiti, Kiran Sankar, Susmita Roy, Renée Lampe, and Alexander Apolonski. 2021. "Detection of Disease-Specific Volatile Organic Compounds Using Infrared Spectroscopy" Engineering Proceedings 8, no. 1: 15. https://doi.org/10.3390/engproc2021008015
APA StyleMaiti, K. S., Roy, S., Lampe, R., & Apolonski, A. (2021). Detection of Disease-Specific Volatile Organic Compounds Using Infrared Spectroscopy. Engineering Proceedings, 8(1), 15. https://doi.org/10.3390/engproc2021008015





