Investigating Sustainable Hydrogen Production via Catalytic Steam Reforming of Ethanol over Stable Commercial Catalysts †
Abstract
1. Introduction
2. Experimental
2.1. Materials
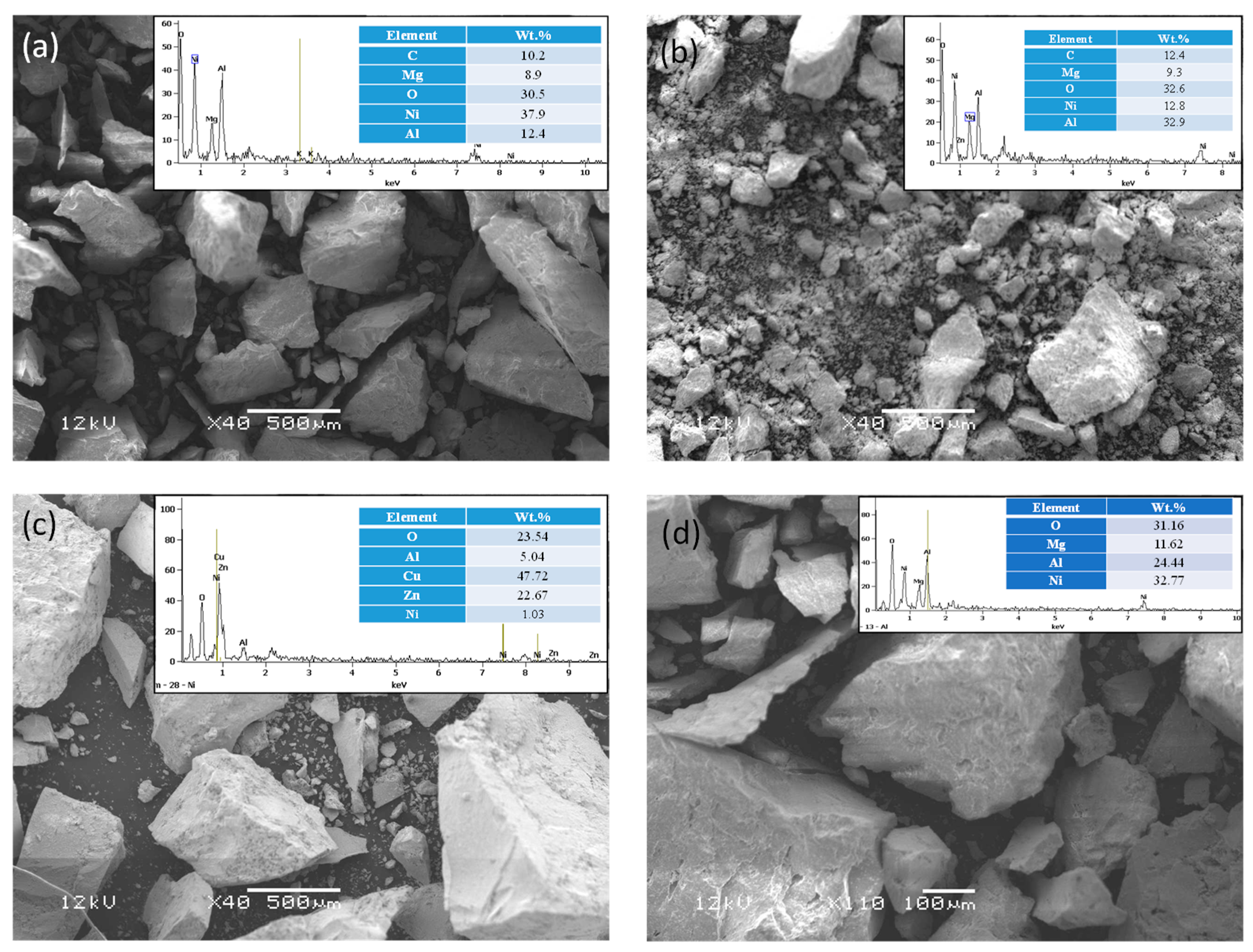
2.2. Catalyst Characterizations
2.3. Catalyst Testing Procedure
3. Results and Discussion
3.1. Effects of Steam-to-Ethanol Ratio
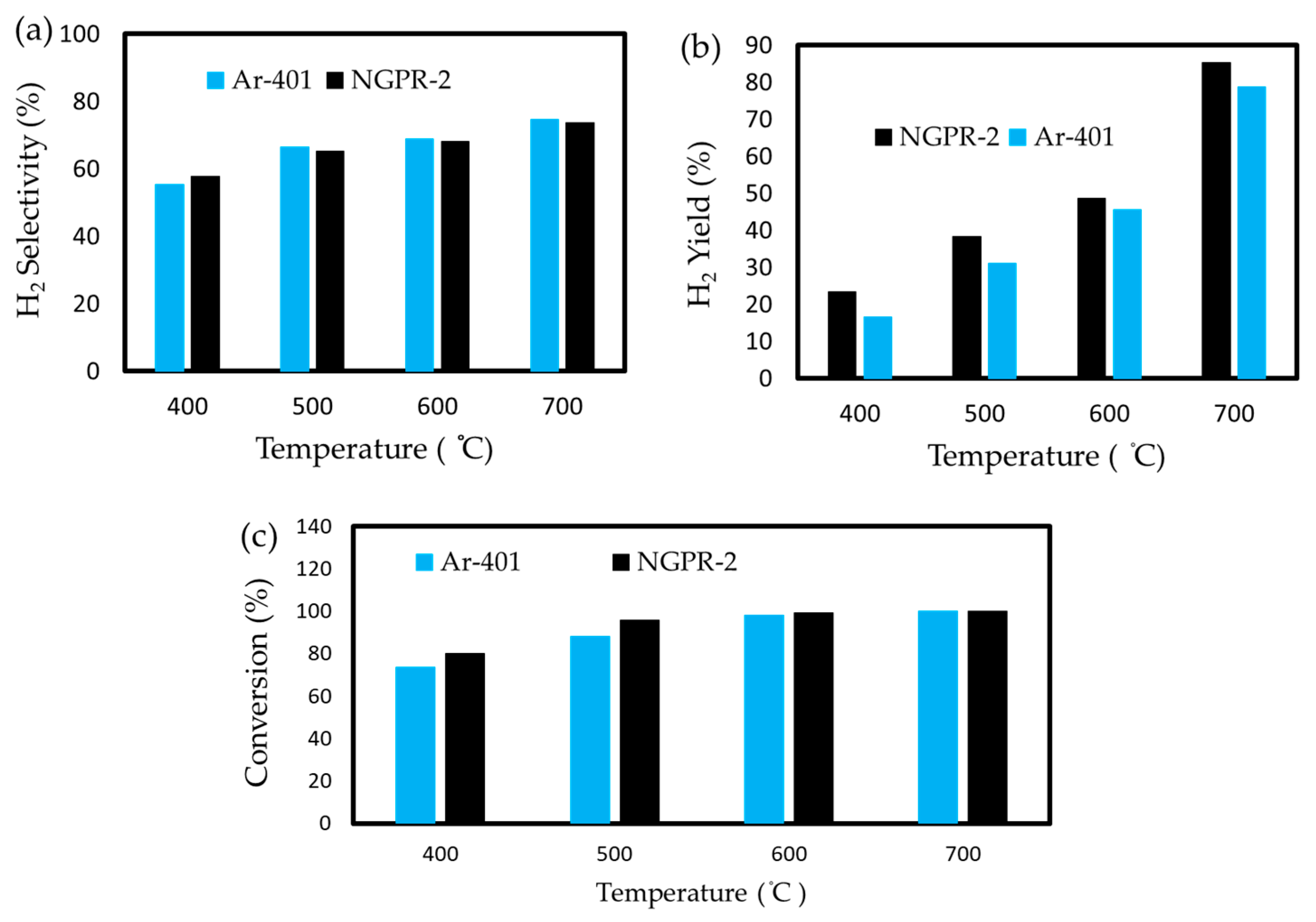
3.2. Effects of Reaction Temperature
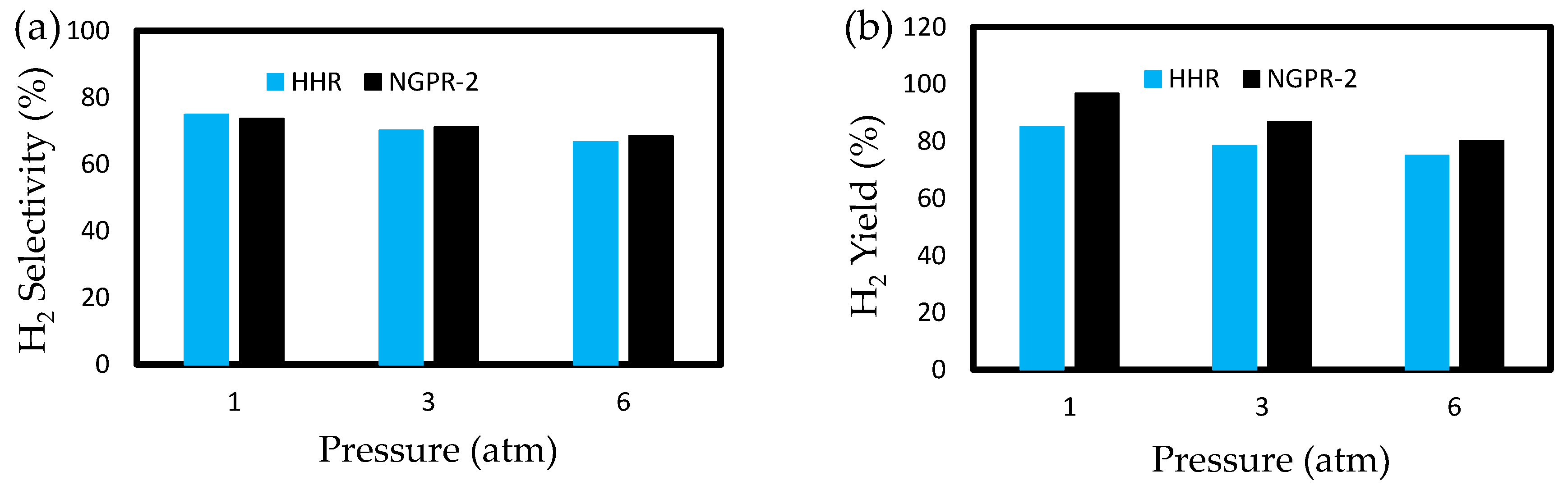
3.3. Effects of Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosha, P.; Ali, F.M.; Ibrahim, H. Recent advances in hydrogen production through catalytic steam reforming of ethanol: Advances in catalytic design. Can. J. Chem. Eng. 2023, 101, 5498–5518. [Google Scholar] [CrossRef]
- Snytnikov, P.V.; Badmaev, S.D.; Volkova, G.G.; Potemkin, D.I.; Zyryanova, M.M.; Belyaev, V.D.; Sobyanin, V.A. Catalysts for hydrogen production in a multifuel processor by methanol, dimethyl ether, and bioethanol steam reforming for fuel cell applications. Int. J. Hydrogen Energy 2012, 37, 16388–16396. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Li, S. Ni-hydrocalumite derived catalysts for ethanol steam reforming on hydrogen production. Int. J. Hydrogen Energy 2022, 47, 24610–24618. [Google Scholar] [CrossRef]
- Jia, H.; Xu, H.; Sheng, X.; Yang, X.; Shen, W.; Goldbach, A. High-temperature ethanol steam reforming in PdCu membrane reactor. J. Membr. Sci. 2020, 605, 118083. [Google Scholar] [CrossRef]
- Cerda-Moreno, C.; Da Costa-Serra, J.F.; Chica, A. Co and La supported on Zn-Hydrotalcite-derived material as efficient catalyst for ethanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 12685–12692. [Google Scholar] [CrossRef]
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A review on ethanol steam reforming for hydrogen production over Ni/Al2O3 and Ni/CeO2 based catalyst powders. Int. J. Hydrogen Energy 2022, 47, 8177–8213. [Google Scholar] [CrossRef]
- Choong, C.K.S.; Huang, L.; Zhong, Z.; Lin, J.; Hong, L.; Chen, L. Effect of calcium addition on catalytic ethanol steam reforming of Ni/Al2O3: II. Acidity/basicity, water adsorption and catalytic activity. Appl. Catal. A Gen. 2011, 407, 155–162. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Wu, Y.-J.; Rodrigues, A.E. Kinetics of Ethanol Steam Reforming for Hydrogen Production; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts-A review. Int. J Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Bepari, S.; Basu, S.; Pradhan, N.C.; Dalai, A.K. Steam reforming of ethanol over cerium-promoted Ni-Mg-Al hydrotalcite catalysts. Catal. Today 2017, 291, 47–57. [Google Scholar] [CrossRef]




| Characterization | Ar-401 | NGPR-2 |
|---|---|---|
| BET Surface Area (m2/g) | 66.47 | 89.9 |
| Pore Size (nm) | 12.06 | 10.47 |
| Pore Volume (cm3/g) | 0.20 | 0.24 |
| Nanoparticle Size (nm) | 90.3 | 65.4 |
| TPR Reduction Temperature (°C) | 295.3 | 306.2 and 828.9 |
| Catalysts | H2 Selectivity (%) | H2 Yield (%) | Ethanol Conversion (%) |
|---|---|---|---|
| Ar-401 | 74.8 | 85.1 | 100 |
| NGPR-2 | 72.6 | 88.7 | 100 |
| NG-608 L | 63.1 | 79.5 | 94.8 |
| MS-901 | 55.5 | 76.8 | 87.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.M.; Rosha, P.; Delfin, K.; Hoagalan, D.; Ibrahim, H. Investigating Sustainable Hydrogen Production via Catalytic Steam Reforming of Ethanol over Stable Commercial Catalysts. Eng. Proc. 2024, 76, 95. https://doi.org/10.3390/engproc2024076095
Ali FM, Rosha P, Delfin K, Hoagalan D, Ibrahim H. Investigating Sustainable Hydrogen Production via Catalytic Steam Reforming of Ethanol over Stable Commercial Catalysts. Engineering Proceedings. 2024; 76(1):95. https://doi.org/10.3390/engproc2024076095
Chicago/Turabian StyleAli, Feysal M., Pali Rosha, Karen Delfin, Dean Hoagalan, and Hussameldin Ibrahim. 2024. "Investigating Sustainable Hydrogen Production via Catalytic Steam Reforming of Ethanol over Stable Commercial Catalysts" Engineering Proceedings 76, no. 1: 95. https://doi.org/10.3390/engproc2024076095
APA StyleAli, F. M., Rosha, P., Delfin, K., Hoagalan, D., & Ibrahim, H. (2024). Investigating Sustainable Hydrogen Production via Catalytic Steam Reforming of Ethanol over Stable Commercial Catalysts. Engineering Proceedings, 76(1), 95. https://doi.org/10.3390/engproc2024076095








