Technical and Environmental Assessment of H2 Production from Cracking Unit Off-Gas: The Terneuzen Case Study †
Abstract
1. Introduction
2. Material and Methods
2.1. Feed Condition and Design Assumptions
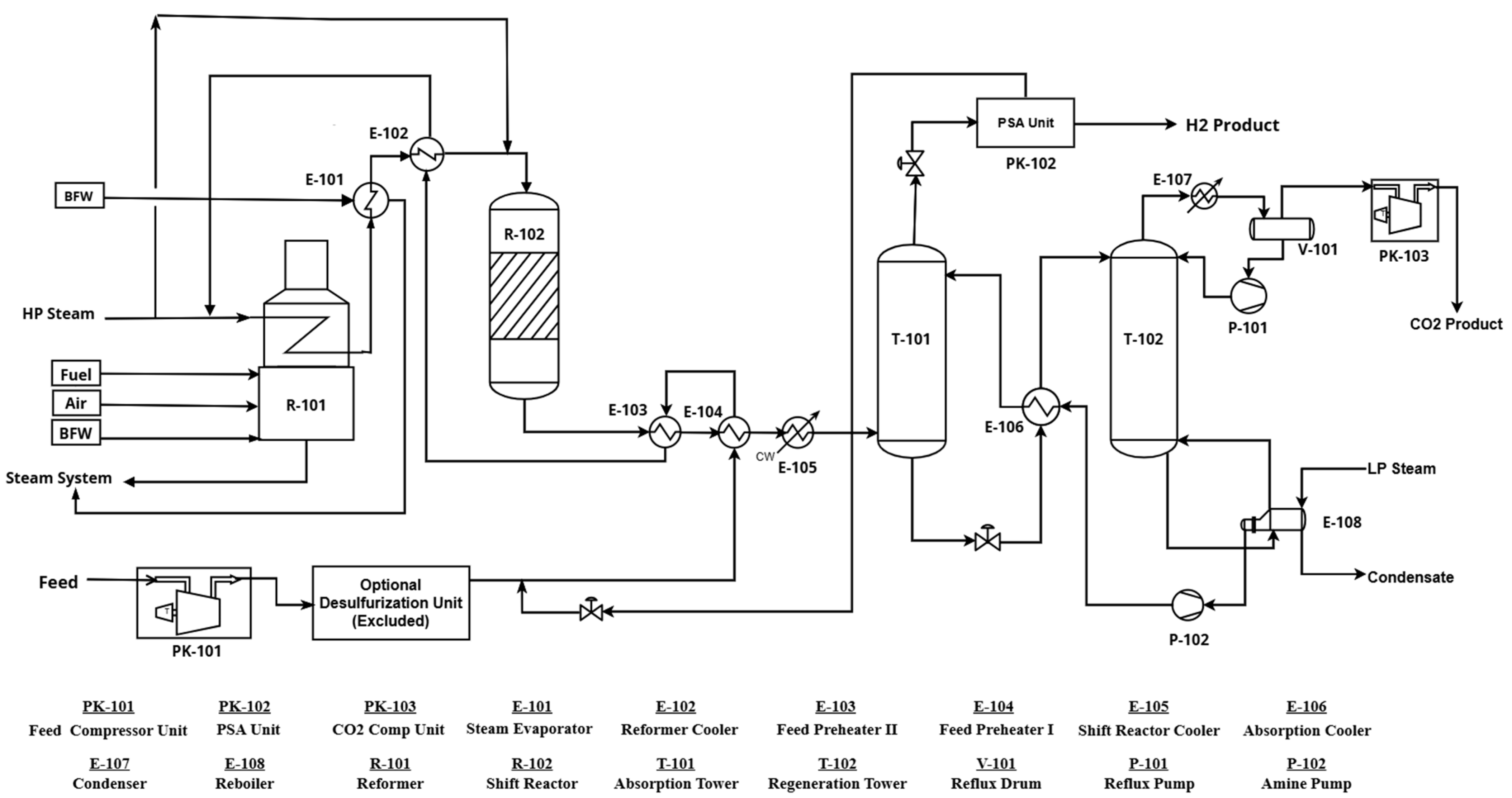
2.2. Process Description
2.3. Process Simulation and Calculation
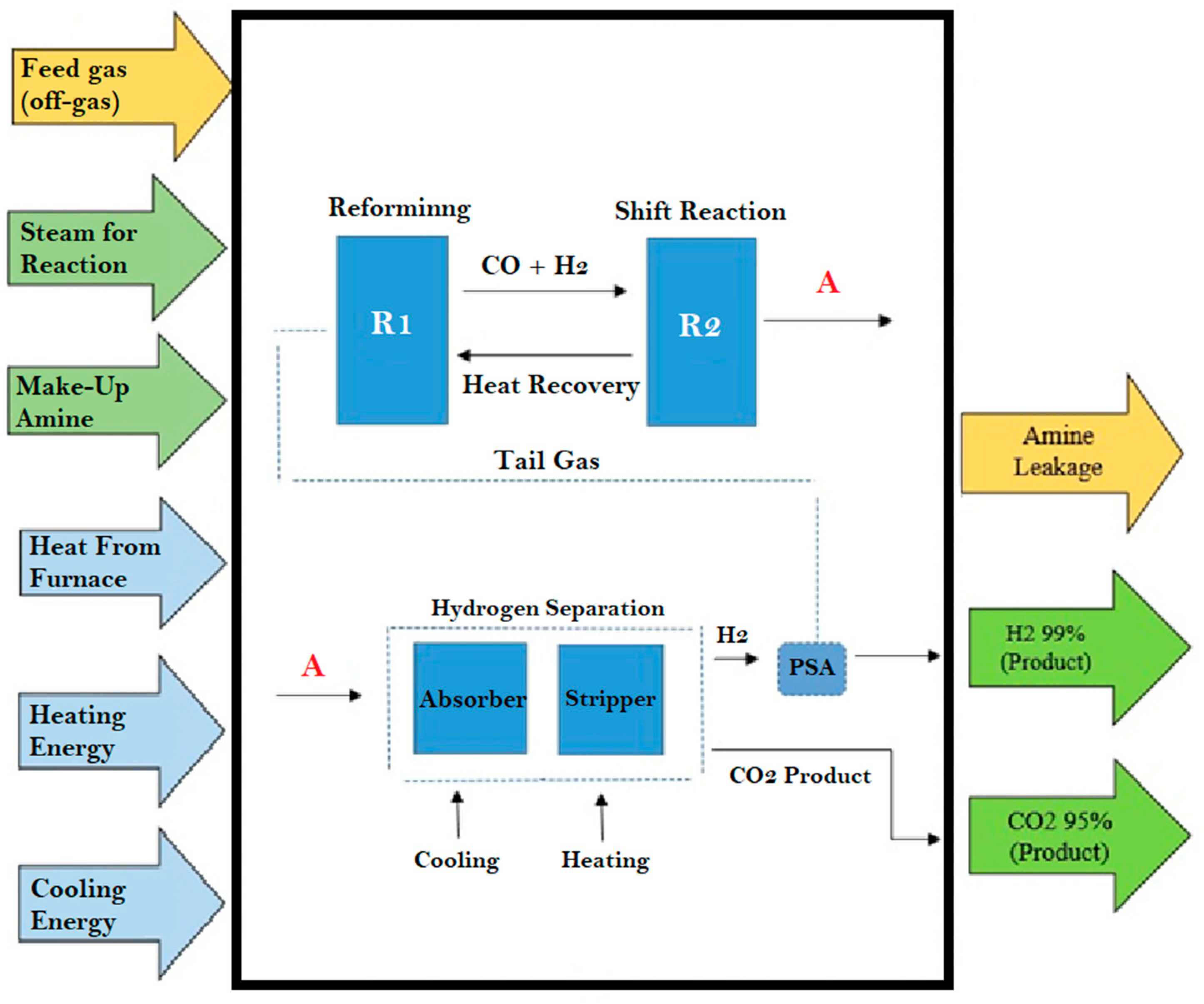
2.4. Life Cycle Assessment
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, R.J. Crude Oil to Chemicals: Industry Development and Strategic Implications. Ind. Arene Chem. 2023, 1, 43–69. [Google Scholar] [CrossRef]
- Andriani, D.; Bicer, Y. A Review of Hydrogen Production from Onboard Ammonia Decomposition: Maritime Applications of Concentrated Solar Energy and Boil-Off Gas Recovery. Fuel 2023, 352, 128900. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Bai, Q.; Liu, Y.; Wang, P.; Xue, S.; Yu, Q.; Li, Q. Road life-cycle carbon dioxide emissions and emission reduction technologies: A review. J. Traffic Transp. Eng. (Engl. Ed.) 2022, 9, 532–555. [Google Scholar] [CrossRef]
- Dermühl, S.; Riedel, U. A comparison of the most promising low-carbon hydrogen production technologies. Fuel 2023, 340, 127478. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Hsu, C.S.; Robinson, P.R. Springer Handbook of Petroleum Technology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Sonnemann, G.; Tsang, M.; Schuhmacher, M. Integrated Life-Cycle and Risk Assessment for Industrial Processes and Products; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Karl, M.; Wright, R.F.; Berglen, T.F.; Denby, B. Worst case scenario study to assess the environmental impact of amine emissions from a CO2 capture plant. Int. J. Greenh. Gas Control. 2011, 5, 439–447. [Google Scholar] [CrossRef]
- Yavary, M.; Ale Ebrahim, H.; Falamaki, C. Competitive adsorption equilibrium isotherms of CO, CO2, CH4, and H2 on activated carbon and zeolite 5A for hydrogen purification. J. Chem. Eng. Data 2016, 61, 3420–3427. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Flow rate | 64,415 kg/h |
| Temperature | 50 °C |
| Pressure | 2 bar |
| Methane, mole% | 90% |
| Ethane, mole% | 10% |
| Sulfur content | 0 |
| Reforming Reactor | Shift Reactor | |||
|---|---|---|---|---|
| Inlet (kmol/h) | Outlet (kmol/h) | Inlet (kmol/h) | Outlet (kmol/h) | |
| Methane | 3323.38 | 424.46 | 424.46 | 424.46 |
| Ethane | 369.26 | 0.01 | 0.01 | 0.01 |
| Water | 15,000 | 11,362.55 | 26,362.55 | 22,853.20 |
| CO | - | 3637.44 | 3637.45 | 128.09 |
| CO2 | - | - | - | 3509.35 |
| MEA | - | - | - | - |
| H2 | - | 10,543.07 | 10,543.08 | 14,052.43 |
| Total | 18,692.65 | 25,967.54 | 40,967.54 | 40,967.54 |
| Gas Inlet | Lean Amine | Gas Outlet | Rich Amine | |
|---|---|---|---|---|
| Methane | 0.01 | - | 0.0224 | 0.0007 |
| Ethane | - | - | - | - |
| Water | 0.56 | 0.84 | 0.0057 | 0.8500 |
| CO | 0.003 | - | 0.0076 | 0.0001 |
| CO2 | 0.086 | - | 0.0689 | 0.0223 |
| MEA | - | 0.16 | 0.0000 | 0.1261 |
| H2 | 0.34 | - | 0.8954 | 0.0008 |
| Component | Feed (kmol/h) | Gas Outlet (kmol/h) | Liquid Outlet (kmol/h) |
|---|---|---|---|
| Methane | 74.58 | 74.58 | 0.001 |
| Ethane | 0.009 | 0.009 | - |
| Water | 92,705.07 | 288.65 | 92,416.42 |
| CO | 10.16 | 10.16 | - |
| CO2 | 2435.48 | 2434.60 | 0.88 |
| MEA | 13,751.38 | - | 13,751.38 |
| H2 | 92 | 92 | - |
| Total | 109,068.67 | 2900 | 106,168.67 |
| Flow | Value | Unit |
|---|---|---|
| Functional Products | ||
| H2 | 1 | kg |
| CO2 (Co-product) | 5.46 | kg |
| Flows | ||
| Steam (Water) For Reaction | 4.47 | kg |
| Cooling Energy | 9.61 | MJ |
| Heating Furnace | 23.09 | MJ |
| General Heating Energy | 21.30 | MJ |
| Electricity | 0.5 | kWh |
| Make-up Amine | 0.6 | kg |
| Indicator for 1 kg H2_Product | Unit | Without Capture (Grey H2) | With Capture (Blue H2) |
|---|---|---|---|
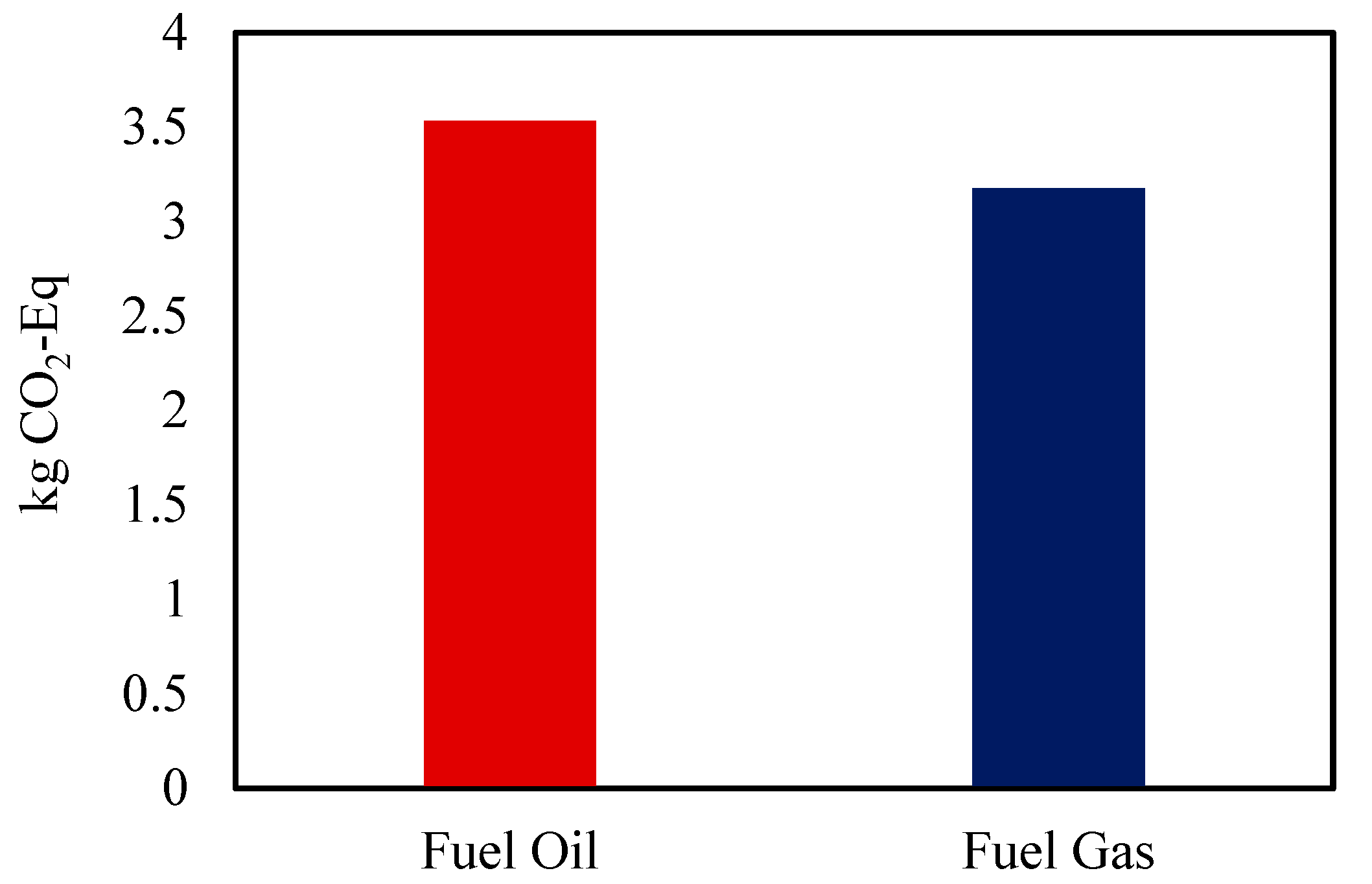
| Greenhouse Emissions | kg CO2-Eq | 8.81 | 3.53 |
| Fossil Fuel Depletion | kg oil-Eq | 4.14 | 2.30 |
| Water Depletion | m3 water | 0.039 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajjadi, M.; Vallès, M.; Nadal, A.; Aljundi, A.; Serra, A.; Osarhiemen, S.; Ibrahim, H. Technical and Environmental Assessment of H2 Production from Cracking Unit Off-Gas: The Terneuzen Case Study. Eng. Proc. 2024, 76, 92. https://doi.org/10.3390/engproc2024076092
Sajjadi M, Vallès M, Nadal A, Aljundi A, Serra A, Osarhiemen S, Ibrahim H. Technical and Environmental Assessment of H2 Production from Cracking Unit Off-Gas: The Terneuzen Case Study. Engineering Proceedings. 2024; 76(1):92. https://doi.org/10.3390/engproc2024076092
Chicago/Turabian StyleSajjadi, Mohammad, Manel Vallès, Arnau Nadal, Ahmed Aljundi, Albert Serra, Sylvester Osarhiemen, and Hussameldin Ibrahim. 2024. "Technical and Environmental Assessment of H2 Production from Cracking Unit Off-Gas: The Terneuzen Case Study" Engineering Proceedings 76, no. 1: 92. https://doi.org/10.3390/engproc2024076092
APA StyleSajjadi, M., Vallès, M., Nadal, A., Aljundi, A., Serra, A., Osarhiemen, S., & Ibrahim, H. (2024). Technical and Environmental Assessment of H2 Production from Cracking Unit Off-Gas: The Terneuzen Case Study. Engineering Proceedings, 76(1), 92. https://doi.org/10.3390/engproc2024076092







