Diffusion Analysis of Flue Gases Through Carbon Nanotube- and Graphene-Reinforced PEBAX Nanocomposite Membranes: A Molecular Dynamic Study †
Abstract
1. Introduction
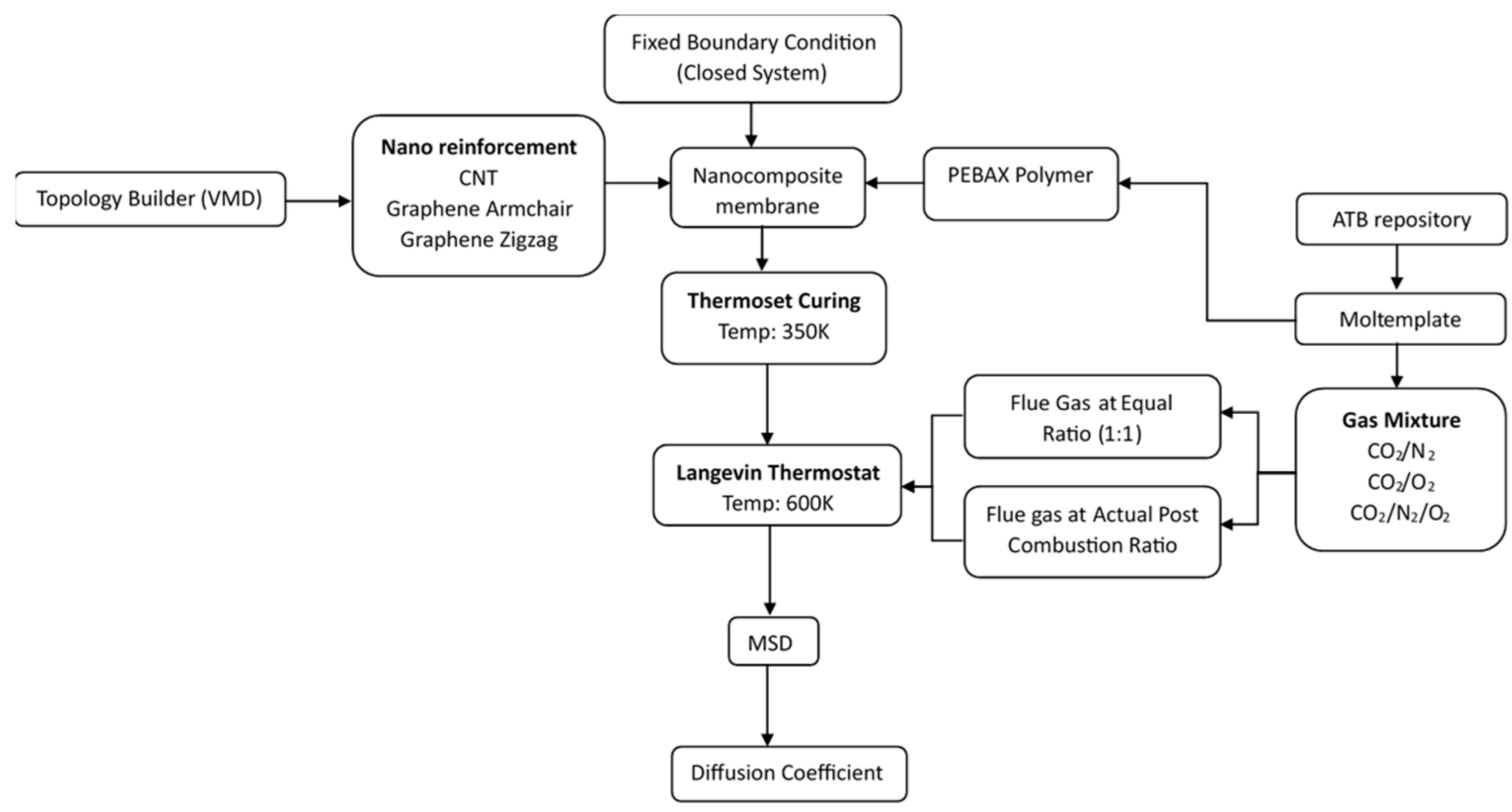
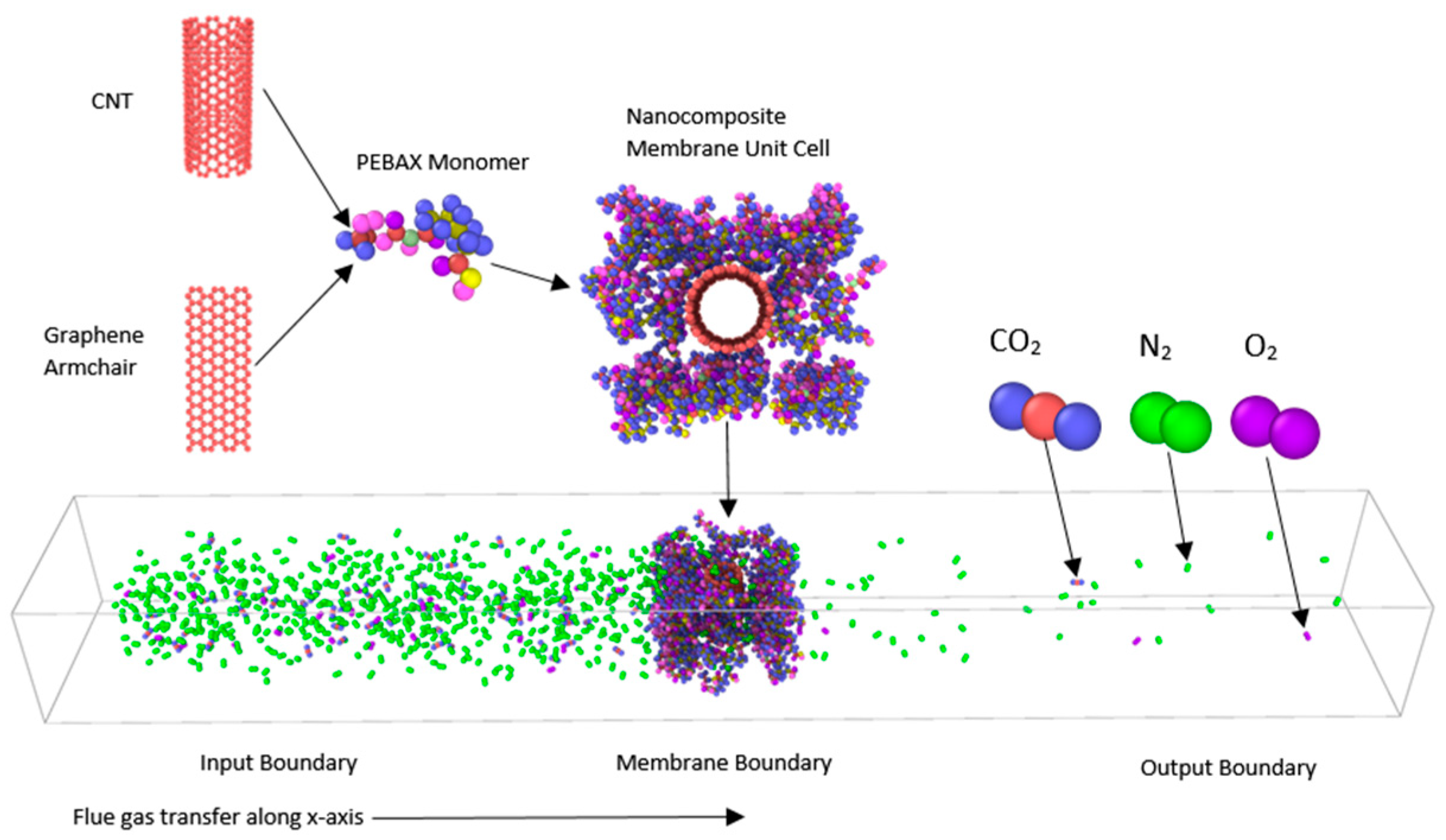
2. Materials and Methods
- Fc is a conservative force of the inter-particle interaction of the molecules;
- Ff is the frictional drag or viscous damping of the particles;
- Fr is the force generated due to the system temperature and random collision of the particle transferring the energy in the process;
- m represents the mass of each particle;
- v is the velocity of the particles in motion;
- kB is the Boltzmann’s constant;
- T represents the system temperature;
- and damp is the damping constant.
3. Results
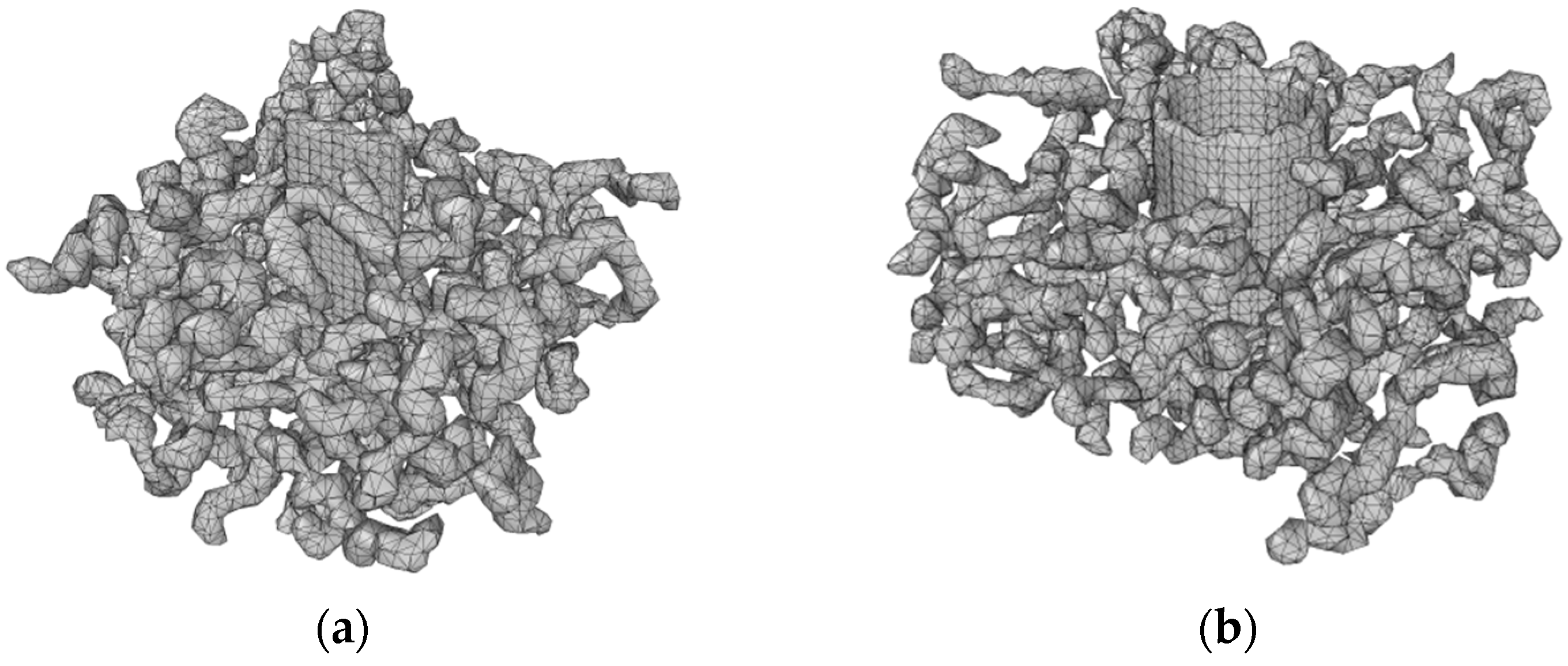
3.1. Surface Mesh Analysis of Nanocomposite
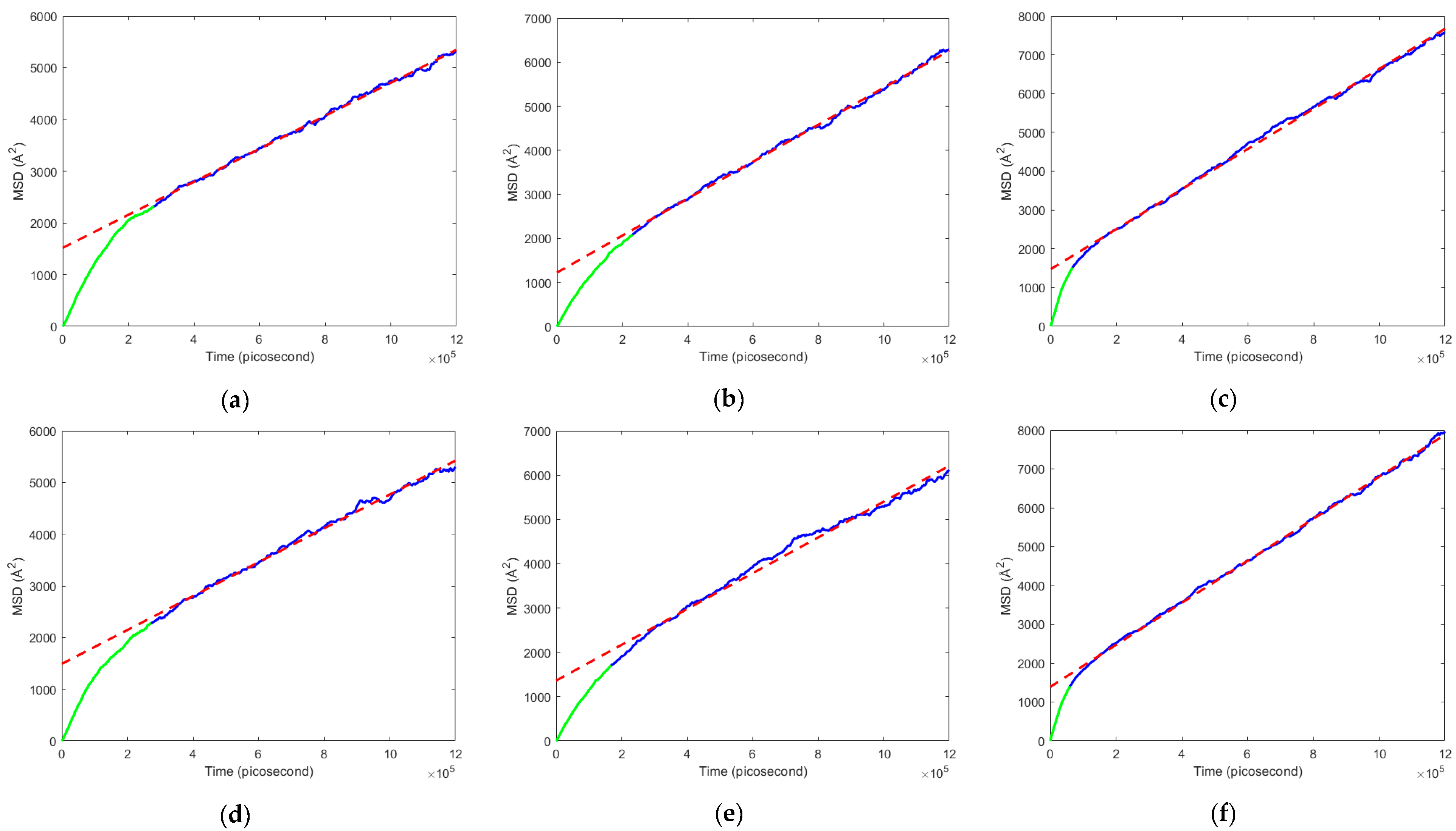
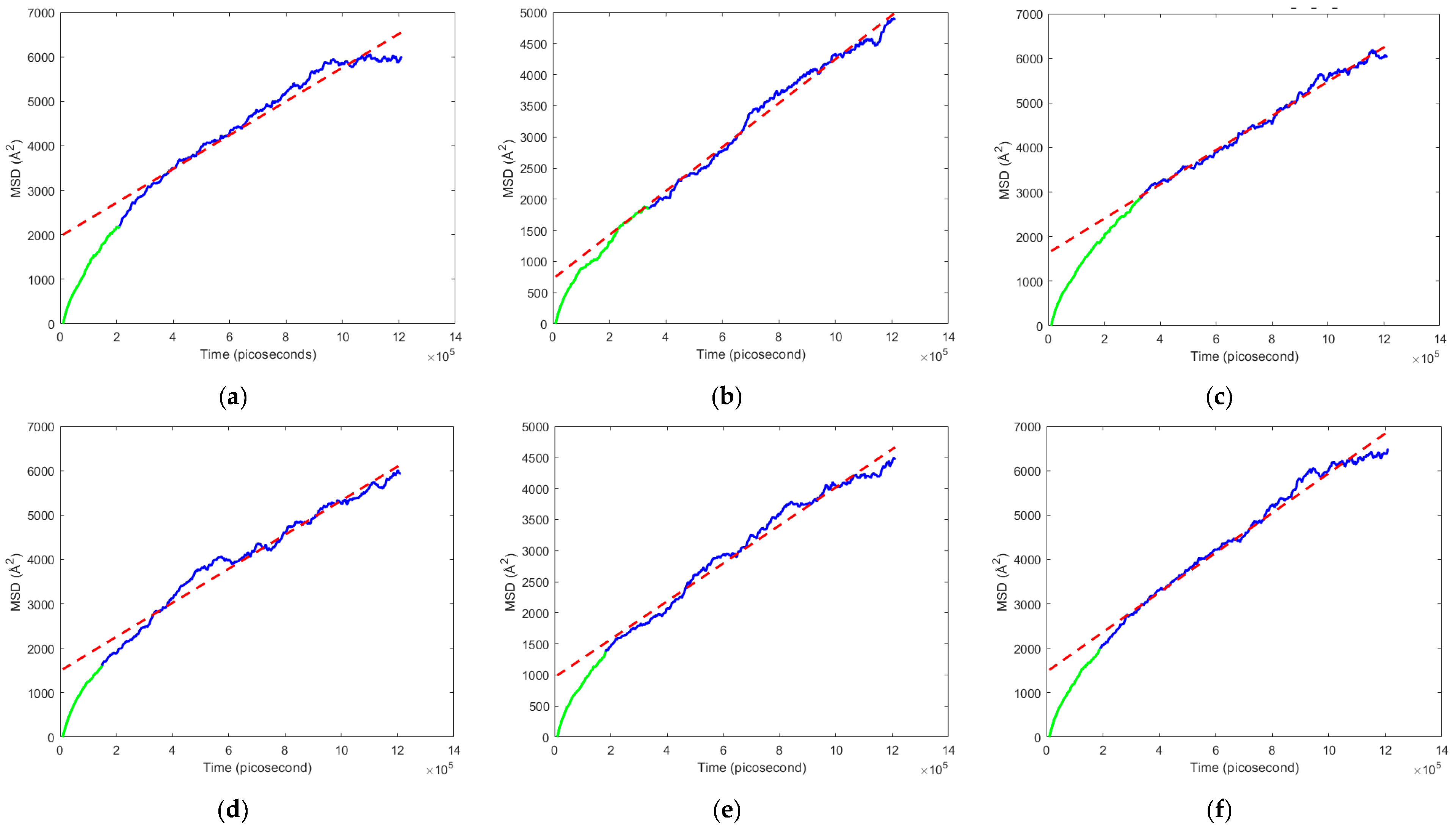
3.2. Mean Squared Displacement and Diffusion Coefficient
4. Conclusions
- Molecular dynamic (MD) simulation is an effective method to determine the diffusion coefficient of flue gas and could be utilized to understand the diffusion mechanisms at the atomic scale under explicit conditions.
- The armchair graphene-reinforced PEBAX nanocomposite showed the highest diffusion coefficient for the N2-enriched flue gas mixture. This is mainly due to the lowest surface area of 13,229 Å2, resulting in a higher region of free volume space, which drove the flue gas with a higher kinematic diameter to diffuse through the membrane following the Knudsen diffusion mechanism.
- The diffusion coefficient for the CNT-reinforced nanocomposite was lower for the O2-enriched flue gas mixture, which is due to the molecular sieve diffusion mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Gupta, Y.; Hellgardt, K.; Wakeman, R. Enhanced permeability of polyaniline based nano-membranes for gas separation. J. Membr. Sci. 2006, 282, 60–70. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Ngoy, J.M.; Wagner, N.; Riboldi, L.; Bolland, O. A CO2 Capture Technology Using Multi-walled Carbon Nanotubes with Polyaspartamide Surfactant. Energy Procedia 2014, 63, 2230–2248. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Wang, Y.; Qiu, Y.; Li, H.; Hua, K.; Li, X.; Ji, J.; Deng, M. High CO2 separation performance of Pebax®/CNTs/GTA mixed matrix membranes. J. Membr. Sci. 2017, 521, 104–113. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. Enhancing Gas Permeation Properties of Pebax® 1657 Membranes via Polysorbate Nonionic Surfactants Doping. Polymers 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Huang, S.; Wu, H.; Li, Y.; Tian, Z.; Jiang, Z. Pebax–PEG–MWCNT hybrid membranes with enhanced CO2 capture properties. J. Membr. Sci. 2014, 460, 62–70. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Peng, K.-J.; Liu, Y.-L.; Deng, L. Pebax/PEG Grafted CNT Hybrid Membranes for Enhanced CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 12226–12234. [Google Scholar] [CrossRef]
- Habibiannejad, S.A.; Aroujalian, A.; Raisi, A. Pebax-1657 mixed matrix membrane containing surface modified multi-walled carbon nanotubes for gas separation. RSC Adv. 2016, 6, 79563–79577. [Google Scholar] [CrossRef]
- Syarif, J.; Badawy, K.; Hussien, H.A. Atomistic simulation of the diffusion behavior in Al-Fe. Nucl. Mater. Energy 2021, 29, 101073. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Li, B.; Wang, S.; Tian, Z.; Yao, G.; Li, H.; Chen, L. Understanding the CO2/CH4/N2 Separation Performance of Nanoporous Amorphous N-Doped Carbon Combined Hybrid Monte Carlo with Machine Learning. Adv. Theory Simul. 2022, 5, 2100378. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; Veld, P.J.I.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.I.; Stelter, D.; Lambert, J.; Saladi, S.M.; Roscioni, O.M.; Ricci, M.; Autin, L.; Maritan, M.; Bashusqeh, S.M.; Keyes, T.; et al. Moltemplate: A Tool for Coarse-Grained Modeling of Complex Biological Matter and Soft Condensed Matter Physics. J. Mol. Biol. 2021, 433, 166841. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, A.; Vermaasand, J.; Braun, E. TopoTools, Release 1.9; Zenodo: Genève, Switzerland, 2022. [Google Scholar]
- Sorichetti, V.; Hugouvieux, V.; Kob, W. Structure and Dynamics of a Polymer–Nanoparticle Composite: Effect of Nanoparticle Size and Volume Fraction. Macromolecules 2018, 51, 5375–5391. [Google Scholar] [CrossRef]
- Kentish, S.E.; Scholes, C.A.; Stevens, G.W. Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications. Recent Pat. Chem. Eng. 2010, 1, 52–66. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]

| Matrix | Reinforcement | Gas Mixture | |||||
|---|---|---|---|---|---|---|---|
| Equal (1:1) (Ratio) | APC (Ratio) * | ||||||
| CO2/N2 | CO2/O2 | CO2/N2/O2 | CO2/N2 | CO2/O2 | CO2/N2/O2 | ||
| PEBAX | CNT | 1:1 | 1:1 | 1:1:1 | 17:3 | 7:3 | 3:16:1 |
| Graphene Armchair | 1:1 | 1:1 | 1:1:1 | 17:3 | 7:3 | 3:16:1 | |
| Equal (1:1) (Ratio) | APC (Ratio) | |||||
|---|---|---|---|---|---|---|
| CO2/N2 | CO2/O2 | CO2/N2/O2 | CO2/N2 | CO2/O2 | CO2/N2/O2 | |
| CNT | 8.87 × 10−7 | 1.17 × 10−6 | 1.43 × 10−6 | 1.05 × 10−6 | 9.8 × 10−7 | 1.07 × 10−6 |
| Graphene Armchair | 9.1 × 10−7 | 1.12 × 10−6 | 1.50 × 10−6 | 1.07 × 10−6 | 8.49 × 10−7 | 1.24 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quader, N.; Muthu, S.D.J.; Kabir, G. Diffusion Analysis of Flue Gases Through Carbon Nanotube- and Graphene-Reinforced PEBAX Nanocomposite Membranes: A Molecular Dynamic Study. Eng. Proc. 2024, 76, 65. https://doi.org/10.3390/engproc2024076065
Quader N, Muthu SDJ, Kabir G. Diffusion Analysis of Flue Gases Through Carbon Nanotube- and Graphene-Reinforced PEBAX Nanocomposite Membranes: A Molecular Dynamic Study. Engineering Proceedings. 2024; 76(1):65. https://doi.org/10.3390/engproc2024076065
Chicago/Turabian StyleQuader, Niaz, S. D. Jacob Muthu, and Golam Kabir. 2024. "Diffusion Analysis of Flue Gases Through Carbon Nanotube- and Graphene-Reinforced PEBAX Nanocomposite Membranes: A Molecular Dynamic Study" Engineering Proceedings 76, no. 1: 65. https://doi.org/10.3390/engproc2024076065
APA StyleQuader, N., Muthu, S. D. J., & Kabir, G. (2024). Diffusion Analysis of Flue Gases Through Carbon Nanotube- and Graphene-Reinforced PEBAX Nanocomposite Membranes: A Molecular Dynamic Study. Engineering Proceedings, 76(1), 65. https://doi.org/10.3390/engproc2024076065








