Abstract
The wastewater of the “Rubizhanskyi” storage facility at the chemical enterprise “Rubizhanskyi Krasitel” (Ukraine) is a complex multicomponent system. The concentration of colored organic substances in the highly mineralized wastewater significantly exceeds the established norms. This work shows the possibility of an almost complete purification of the multicomponent wastewater using a carbon sorbent. The treatment of wastewater was carried out using the sorption method with carbon adsorbents KARBON™ (Institute for Sorption and Problems of Endoecology, Kyiv, Ukraine) and ZL-302, which are micro-mesoporous materials, with specific surface areas of 1730 and 1523 m2/g, respectively. The purification efficiency of the wastewater containing aromatic compounds by the carbon adsorbents KARBON™ and ZL-302 achieved 99.99% and 94.4%, respectively. The adsorption capacity of the carbon adsorbent KARBON™ with respect to aniline was 0.47 g/g, and that of commercial coal ZL-302 was 0.45 g/g. Therefore, the carbon adsorbent KARBON™ is a promising material for the successful purification of wastewater from chemical enterprises contaminated with aromatic compounds.
1. Introduction
The storage facilities of chemical enterprises contain wastewater with many different components. They contain insoluble impurities, suspensions, and dissolved substances of a mineral and organic nature (surfactants, oxidants, and dyes) and have a specific color and medium acidity (pH) from 4 to 12.5 [1]. The modern organic and organometallic dyes which are produced by the “Rubizhansky Krasitel” enterprise (Rubizhne, Luhansk region, Ukraine) have a low capacity for biodegradation, as well as resistance to the chemical and temperature effects of the environment [2]. For many years, the waste from this enterprise, which produces dyes, bleaches, synthetic resins, plant protection products, plasticizers, and pigments, was discharged into the wastewater reservoir “Rubizhanskyi” (Figure 1). There have been different degrees of human-made impact on the nearby area because of the tailing storage facility (TSF) “Rubizhansky Krasitel” situated close to 1 km from the Siverskyi Donets River and 1.3–3.4 m from groundwater level. Up to 1.7 million m3 of waste have been produced, consisting of 34 types of chemical products [3]. An analysis of the salt composition of the Siverskyi Donets River in 2019 showed that the water was dominated by sulfates, chlorides, hydrocarbonates, and some elements (Ni, Zn, Mn, Cu, Al, etc.); the water was quite mineralized [3]. Currently, the reservoir comprises heavily polluted, colored industrial wastewater containing aromatic sulfonic acids, nitrobenzene, chlorobenzene, phenol, and mineral salts. The phenol (carbolic acid or phenolic acid, C6H5OH) concentration exceeded the maximum permissible amount by 67–85 times, while aniline (benzenamine, C6H5NH2) exceeded the maximum permissible concentration by 19–22 times [4].

Figure 1.
Sections of the highly mineralized waste storage facility “Rubizhanskyi”. Sections 1 and 5 contain industrial wastewater, sections 2 and 3 contain atmospheric precipitation, and Section 4 contains residual activated sludge from sewage treatment plants in the city of Rubizhne.
Fighting is currently underway in the Luhansk region, which makes it impossible to monitor the groundwater. However, this may lead to emergencies in the “Rubizhanskyi” wastewater reservoir, with contaminated sewage entering the surface and underground waters of the city of Rubizhne. Therefore, establishing effective cleaning methods for carrying out cleanup activities in the region is urgent, and this will be an important environmental task to undertake in peacetime.
One of the most efficient methods of industrial wastewater purification is the adsorption method, because it is rapid, inexpensive, simple, and requires low investment. It ensures the almost complete removal and/or destruction of pollutants. Clay minerals, montmorillonite and palygorskite, modified by cationic surfactants were used for the removal of dyes from wastewater of the “Rubizhansky” tailing storage facility [5]. The dye removal efficiency observed in the wastewater from the “Rubizhansky” tailing storage facility that was treated with the modified clays was 91.6% and 96.5%, respectively.
Activated carbon has been used in sorption technologies due to its large specific surface area, strength, selectivity, and high adsorption capacity for various pollutants, especially aromatic compounds [6,7]. Corncob [8], dogwood seed [9], spent coffee grounds [10], rice husk [11], orange peel [12], walnut shell [13], sugarcane bagasse [14], aloe vera leave [15], watermelon rind [16], and many other different products have been used as sources of active carbon by many researchers.
Activated carbon from Chemviron Carbon (Feluy, Belgium), Donau Carbon (Frankfurt am Main, Germany), Calgon Carbon (Pittsburgh, USA), and the Kureha Corporation (Tokyo, Japan) has found the greatest use globally [17], where the starting materials of the carbon adsorbents are lignite, coconut shell, and nut shell.
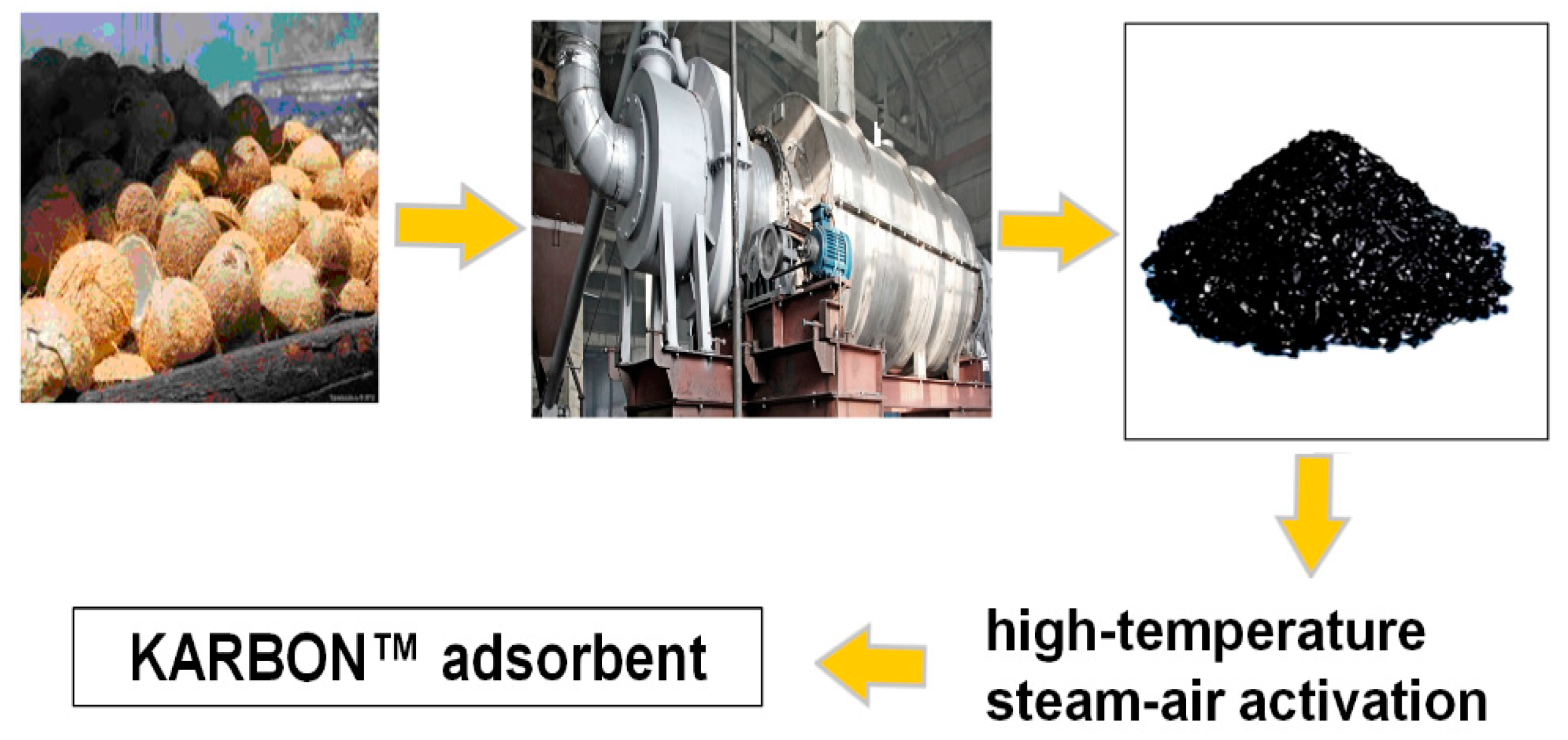
At the Institute of Sorption and Problems of Endoecology in the National Academy of Sciences of Ukraine, the carbon adsorbent KARBON™ was synthesized from the commercial coconut charcoal AquaCarb 607C (Chemviron Carbon, Belgium) by additional high-temperature (t = 850 °C) steam–air activation (0.5–5.0%, 0.5–3 h) method [18]. The purpose of this work was to show the possibility of the sorption purification of wastewater from the “Rubizhanskyi” storage tank from organic compounds with carbon adsorbents (based on their physical-chemical characteristics) and to compare their the efficiency of purification and adsorption capacity with similar porous structure sorbents.
2. Methods
Wastewater samples were taken from the fifth section of the “Rubizhansky” reservoir because wastewater from this section of the tailing pond is the most dangerous for the environment. The total organic carbon (TOC) content was determined by the catalytic combustion method using a Shimadzu TOC-V CSN device (Kyoto, Japan). The determination of total dissolved solids, as well as the content of inorganic and organic compounds, total alkalinity, and hydrocarbon ions content in the wastewater, was carried out according to the established methods [19]. The acidity of the wastewater was measured using a 781 pH/Ion Meter (Metrohm, Herisau, Switzerland).
For wastewater treatment, the carbon adsorbent KARBON™ (the synthesis scheme is presented in Figure 2) and a commercial coal ZL-302 (Huzhou Beigang&Exp.co, LTD, China) were used. Samples of the KARBON™ adsorbent with a bulk density of 0.35 g/cm3 and a particle size of 0.25–0.63 mm, as well as the activated carbon ZL-302 series 041206 with a bulk density of 0.345 g/cm3 and a particle size of 0.06–0.12 mm, were selected for research.
Figure 2.
Scheme for KARBONTM production from coconut shells.
The porous structure of the carbon materials used was studied by the low-temperature nitrogen adsorption method using a gas surface analyzer Quantachrome NOVA 2200 (Surface Area and Pore Size Analyzer, Boynton Beach, FL, USA). The specific surface area (Ssp, m2/g), pore volume (V, cm3/g), pore radius (r, nm), and size distribution of the pore volumes (dV/dlog(r)) were calculated using the BET, BJH, and DFT methods using the ASiQwinTM V 3.0 software. The morphology of the KARBON™ adsorbent was studied by scanning electron microscopy (SEM) using a JEOL JSM-6700F microscope (Tokyo, Japan).
Sorption removal was carried out under static conditions at 25 °C, with the continuous shaking of the samples for 4 h. The suspended particles were removed from the wastewater by centrifugation before the main experiments. The weights of the sorbent were 0.1, 0.5, and 1 g, and the volume of wastewater was 0.025 L (solid/liquid = 4, 20, and 40, respectively). After separating the solid and liquid phases, the optical density of the solution was determined on a Shimadzu UV-2450 spectrophotometer (Kyoto, Japan) in quartz cuvettes (l = 10 mm) at wavelengths of 200−450 nm. The efficiency of the removal of organic substances (R, %) was calculated by the difference between the initial optical density of the solutions (D0) and the optical density after establishing the adsorption equilibrium (Da), as shown in Equation (1).
R = (D0 − Da)D0 * 100
The initial (Cinit., g/L) and equilibrium (Ceq., g/L) concentrations of aniline were found according to [20]. The adsorption capacity (A, g/g) of the adsorbents with respect to aniline was calculated using the following formula (2):
where V is the solution volume, L; and m is the sorbent mass, g.
A = (Cinit. − Ceq.) * V/m
3. Results and Discussion
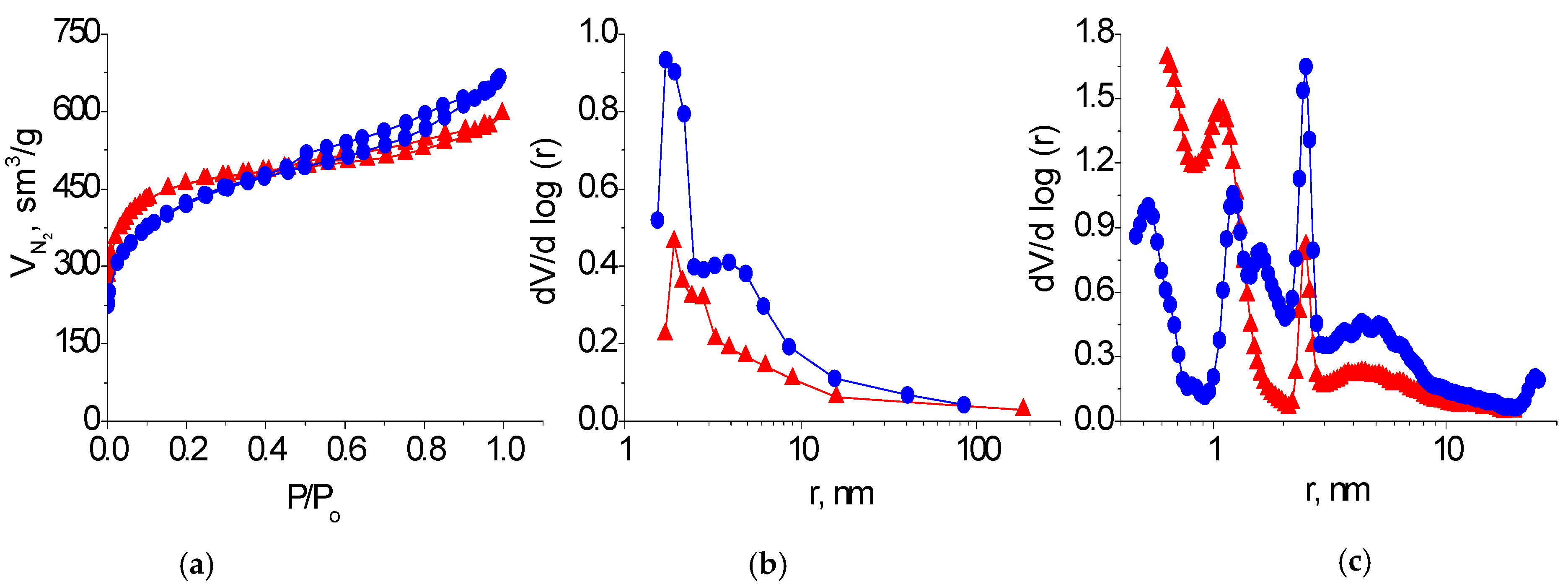
The nitrogen adsorption/desorption isotherms of the KARBON™ and ZL-302 carbon adsorbents belong to type I isotherms with a hysteresis loop of type H4 according to the IUPAC classification [21] (Figure 3a). A sharp increase in the amount of absorbed nitrogen at low relative pressure and the presence of hysteresis loops on the isotherm at medium and high pressure indicate that these adsorbents are micro-mesoporous materials. It can be seen from the distribution curves of the pore volumes by size that carbon adsorbent KARBON™ contains micropores with a radii of 0.6−1.0 nm and mesopores with a radii of 1−1.5 and 2−3 nm. The number of mesopores of different sizes increases in the porous structure of the ZL-302 coal (Figure 3b,c; Table 1).
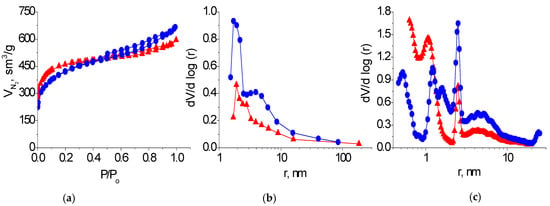
Figure 3.
Nitrogen adsorption/desorption isotherms (a) and differential pore size distribution curves obtained by BJH (b) and DFT (c) methods on samples of KARBON™ (▲) and ZL-302 (●) adsorbents.

Table 1.
Parameters of the porous structure of the adsorbents studied.
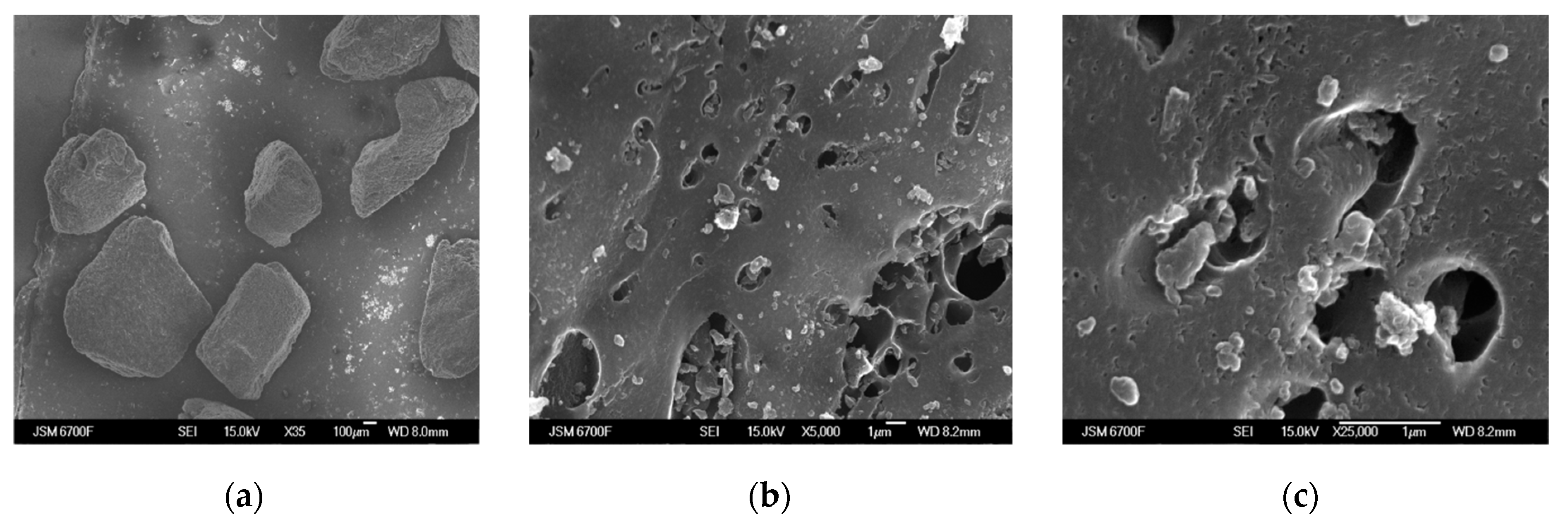
The morphology of the carbon sorbents KARBON™ and ZL-302 is presented in Figure 3. It can be seen from the SEM photographs that the carbon adsorbent KARBON™ has particles of irregular shape with a size of 0.2−0.4 mm (Figure 4a). At a magnification of 5000 and 25,000 times, surface transport pores are visible, the size of which depends on the bulk density of the KARBON™ adsorbent [22] (Figure 4b,c).
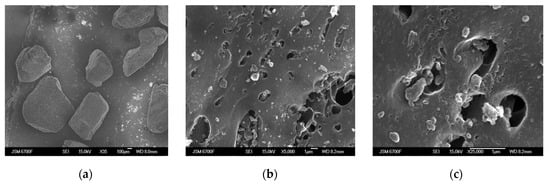
Figure 4.
SEM photographs of KARBON™ carbon adsorbent with magnification of 35 (a), 5000 (b), and 25,000 times (c).
The wastewater from the “Rubizhanskyi” reservoir was thick, dark brown in color, and contained a large amount of suspended particles (Figure 5). The TOC content of the pretreatment waters was found to be 1130 ppm at a 1000-fold dilution. The total content of organic and inorganic substances in terms of dry residue was 98.5 g/L, while calcined residue (mineral substances) was 79.6 g/L. Thus, the content of organic substances was 18.9 g/L. The total alkalinity of the initial wastewater was 0.16 g-eq/g, and the content of hydrocarbon ions was 9.76 g/L, pH = 4.

Figure 5.
Water sample from section 5 and dry residue.
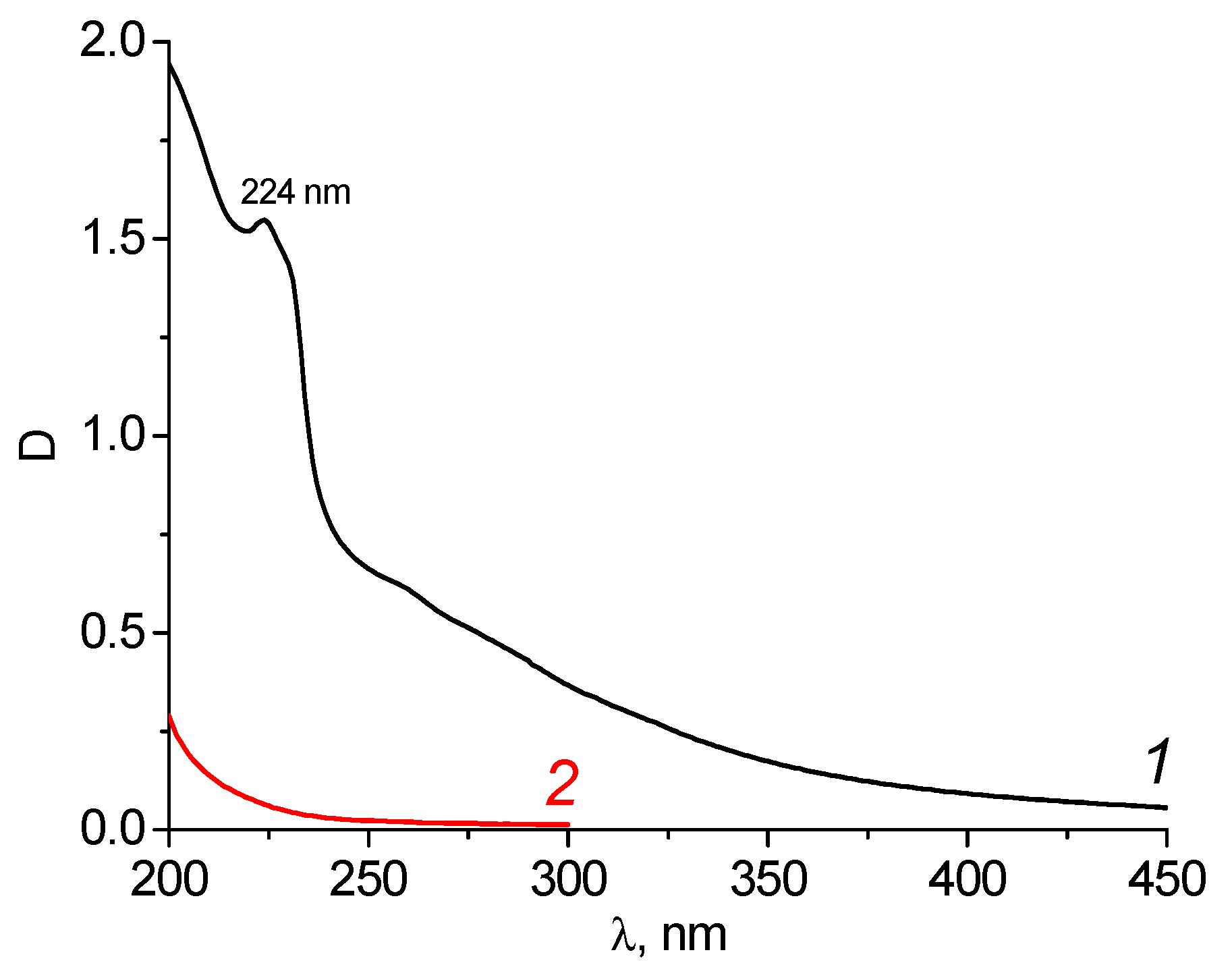
In the UV spectrum of the initial wastewater, a band with a maximum of 224 nm was observed (Figure 6, curve 1). This may indicate the presence of both aniline and phenol (maximum absorption of aniline at 234/288 nm, phenol at 210.5/270 nm; solvent water, pH = 3) [23].
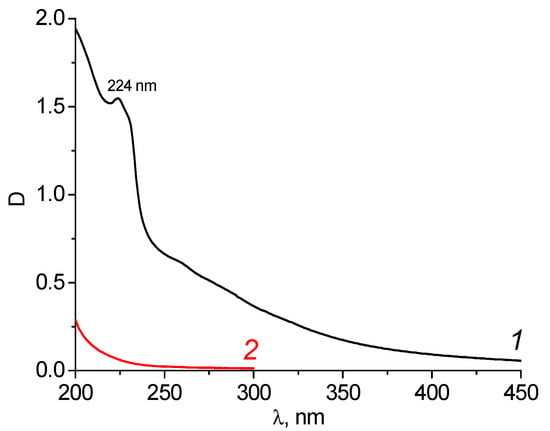
Figure 6.
Spectra of the wastewater of the “Rubizhanskyi Krasitel” enterprise before (dilution 1000 times) (1) and after purification by carbon adsorbent KARBON™ (2).
It was established that the best conditions for wastewater purification from the “Rubizhanskyi” TFS would be a weight of 1.0 g of adsorbent and a volume of water of 0.025 L (component ratio 40) since these result in the complete purification of wastewater from all toxicants (Figure 6, curve 2). After treatment, the wastewater completely loses its color. At the same time, the pH value of the solutions increases to 6.0–6.5 for both the carbon adsorbents. The efficiency of water purification from the aromatic compounds by the carbon adsorbent KARBON™ was 99.99%. This exceeded the efficiency of wastewater treatment at the “Rubizhanskyi Krasitel” enterprise using both the commercial coal ZL-302 (94.4%) and the clays modified with surface-active substances [5].
The aniline contents we found using the works [19,20] is the same. Traces of phenol were detected; its content could not be quantified. The adsorption capacity of the carbon adsorbent KARBON™ in relation to aniline was 0.47 g/g and that of commercial coal ZL-302 was 0.45 g/g.
Since, as noted, the wastewater of the “Rubizhanskyi Krasitel” enterprise is a multi-component system with a significant content of both organic and inorganic substances, the indicated amount of sorbent required for complete purification includes not only the removal of phenol and aniline but also all other components. The absorption of phenol by carbon samples may be determined by the π–π interaction of electrons of the phenol ring and intraframework graphene, the formation of electron–donor–acceptor complexes of phenol with oxygen groups, and/or the formation of hydrogen bonds with the OH groups of phenol [24,25]. The specific adsorption of aniline can occur due to strong hydrogen bonding of the amino group or the donor–acceptor p-electron interactions of the aniline molecules with surface oxygen compounds of both acidic and basic types, as well as the dipole–dipole interaction of weak hydrogen bonds [26].
4. Conclusions
The TFS of the “Rubizhansky Krasitel” enterprise has a significant technogenic impact on the surrounding areas and poses an environmental threat, especially now with the hostilities. Determining the optimal ways to purify highly mineralized multicomponent waters to prevent their entry into the environment, namely into the groundwater and the aquatic environment of the Siverskyi Donets River, is an important task for ecologists. Aniline and phenol identified in the wastewater, as well as other components of the system, were effectively removed (removal rate 99.99%) using the KARBON™ carbon adsorbent. Therefore, taking into account the availability of raw materials, the simplicity of the production method, as well as the high adsorption capacity, the KARBON™ carbon adsorbent can be recommended for the purification of wastewater from chemical enterprises of this type, in particular the TFS of the “Rubizhansky Krasitel” enterprise.
Author Contributions
Conceptualization, I.F.; methodology I.F.; software, I.F.; validation, I.K. and I.F.; formal analysis, I.F.; investigation, I.K.; writing—original draft preparation, I.F. and I.K.; writing—review and editing, I.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors express their sincere gratitude to the researcher of ISPE of the National Academy of Sciences of Ukraine M.M. Tsyba for measuring coal samples by the method of low-temperature nitrogen adsorption.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nikolaieva, I.; Lenko, H.; Lobodzinskyi, O. Donbas Tailings Storage Facilities. Available online: https://www.osce.org/files/f/documents/2/5/456844.pdf (accessed on 5 March 2025).
- Hao, O.J.; Kim, H.; Chang, P.-C. Decolorization of Wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Mikhalkova, N.; Kononenko, A.; Udalov, I. Analysis of the influence of technogenic facilities of the Lysychansk-Rubizhne industrial hub on the ecological state of the natural environment. Visnyk V.N. Karazin Kharkiv Natl. Univ. Ser. Geol. Geography. Ecol. 2022, 56, 225–239. [Google Scholar] [CrossRef]
- Nikolaieva, I.; Lenko, A.; Averin, D.; Lobodzinsky, A. Doslidjennya Potochnogo Stanu Chvostoschovyshch u Donerskii ta Luganskii Oblastyach. Available online: https://www.osce.org/files/f/documents/9/9/486259.pdf (accessed on 5 March 2025). (in Russian).
- Farbun, I.A.; Kovalchuk, I.A.; Khalyavka, T.A.; Tsyba, M.M.; Camychan, S.V. Organic Pollutants Removal from Wastewater in Rubizhne City, Ukraine. In Proceedings of the XVI International Scientific Conference «Monitoring of Geological Processes and Ecological Condition of the Environment», Kyiv, Ukraine, 15–18 November 2022; pp. 1–5. [Google Scholar]
- Marsh, H.; Rodrigues-Reinoso, F. Activated Carbon; Elsevier Science and Technology Books: Oxford, UK, 2006; 536p. [Google Scholar]
- Ho, S. Removal of Dyes from Wastewater by Adsorption onto Activated Carbon: Mini Review. J. Geosci. Environ. Prot. 2020, 8, 120–131. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puiy, A.M. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Sych, N.V.; Vikarchuk, V.M.; Kupchyk, L.A.; Fedorishin, A.S.; Kravchenko, O.V. Advances in B(III) Removal by Adsorption on Nanoporous Carbon of Lignocellulosic Origin and Its Surface Modified Analogue. Nanosistemi Nanomater. Nanotehnol. 2021, 19, 629–638. [Google Scholar]
- Sych, N.V.; Kotynskaya, L.I.; Vikarchuk, V.M.; Tsyba, M.M.; Kovtun, M.F. Sorption of Phenolic Acids on Powdered Carbon Prepared by Complex Processing of Spent Coffee Grounds. Phys. Chem. Water Treat. Process. 2020, 42, 88–93. [Google Scholar] [CrossRef]
- Khu, L.V.; Thuy, L. Preparation of Pore Size Controllable Activated Carbon from Rice Husk Using Dual Activating Agent and Its Application in Super Capacitor. J. Chem. 2019, 5, 1–11. [Google Scholar]
- John, K.; Lin, S.; Amit, K.; Zhao, Y.; Choi, J.; Song, M.; Cho, C.; Yun, Y. Evaluation of Orange Peel Derived Activated Carbons for Treatment of Dye Contaminated Wastewater Tailings. Environ. Sci. Pollut. Res. 2020, 27, 1053–1068. [Google Scholar]
- Yu, Q.; Ning, P.; Li, M.; Tang, X. Characterization of Metal Oxide Modified Walnut Shell Activated Carbon and Its Application for Phosphine Adsorption: Equilibrium, Regeneration and Mechanism Studies. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 487–495. [Google Scholar] [CrossRef]
- Mahanta, V.; Raja, M.; Kothandaraman, R. Activated Carbon from Sugarcane Bagasse as a Potential Positive Electrode Catalyst for Vanadium Redox Flow Battery. Mater. Lett. 2019, 247, 63–66. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Sudhan, N.; Sathish, M.; Ilayaraja, N. Aloe Vera Derived Activated High Surface Area Carbon for Flexible and High Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Razuan, R.; Jimmy, N.; Lee, D. Adsorption and Mechanism Study for Methylene Blue Dye Removal with Carbonized Watermelon (Citrullus lanatus) Rind Prepared via One Step Liquid Phase H2SO4 Activation. Surf. Interfaces 2019, 16, 76–84. [Google Scholar] [CrossRef]
- Activated Carbon Market Size, Share & COVID-19 Impact Analysis, by Type (Powdered, Granular, and Others), by Application (Water Treatment, Air & Gas Purification, Food & Beverage, Pharmaceutical & Healthcare Treatment, and Others), and Regional Forecast, 2024–2032. Available online: https://www.fortunebusinessinsights.com/activated-carbon-market-102175 (accessed on 17 February 2025).
- Trykhlib, V.A.; Strelko, V.V. Method of Producing of Micro- and Mesoporous Carbon Adsorbent. U.S. Patent 109548, 25 August 2016. [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Bridgewater, L., Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; APHA-AWWA-WEF: Washington, DC, USA, 2012; 1496p. [Google Scholar]
- Bauer, K.H.; Moll, H. Die organische Analyse., Geest & Portig: Leipzig, Germany, 1954; p. 151. Available online: https://www.zvab.com/organische-Analyse-Bauer-Karl-Hugo-Heinrich/22631011246/bd (accessed on 17 February 2025).
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Farbun, I.A.; Trykhlib, V.A.; Tsyba, M.M. Characteristics of the sorption of aromatic and heterocyclic amino acids by activated coconut carbons with different bulk density. Vopr. Khimii I Khimicheskoi Tekhnologii 2020, 129, 125–133. [Google Scholar]
- Dawson, R.M.C.; Elliott, D.C.; Elliott, W.H.; Jones, K.M. Data for Biochemical Research, 3rd ed.; Oxford University Press: Oxford, England, 1986; 580p. [Google Scholar]
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption. Energy Fuels 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Tamarkina, Y.V.; Anishchenko, V.M.; Red’ko, A.M.; Kucherenko, V.O. Alkali activated coals. Microporous structure and capability to adsorb phenol compounds. Chem. Phys. Technol. Surf. 2022, 13, 111–124. [Google Scholar]
- Zuo, L.; Wenzhe, S.; Taihong, S.; Lv, C.; Yao, J.; Liu, J.F.; Weng, Y. Adsorption of aniline on template-synthesized porous carbons. Microporous Mesoporous Mater. 2014, 200, 174–181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).