Abstract
The Food and Agriculture Organization (FAO) reports that 1.3 billion tons of food are wasted annually, with 42% of this waste attributed to fruits and vegetables. This problem is often due to distribution challenges and long supply chains, leading to spoilage before reaching consumers. To address this, advancements in cold storage have focused on using phase-change materials (PCMs) to enhance preservation. This study explored improving portable cold storage using smaller PCM packs in various configurations. The selected PCMs were a 10% aqueous KCl solution and a 10% aqueous PEG6000 solution, each stored in 200 g HDPE packs. The packs were arranged in six configurations within a portable cold storage unit measuring 340 mm × 255 mm × 285 mm to investigate temperature distribution and heat transfer. The best performance was achieved when the packs were placed on the upper side of the storage unit, ensuring even temperature distribution with a standard deviation of ±0.27 °C. The KCl-based PCM was most effective at maintaining the desired cold temperature for 7.2 h, while the PEG6000-based PCM performed well at slightly higher temperatures, which could still be beneficial depending on storage needs.
1. Introduction
According to a 2022 report by the Food and Agriculture Organization (FAO), annually, approximately 1.3 billion tons of food are wasted globally, with fruits and vegetables constituting about 42% of this total [1,2]. This substantial waste largely results from logistical challenges and extended supply chains, which often cause food to spoil before it can be consumed [3]. Vegetables and fruits need to be stored at a specific temperature, usually lower than room temperature, to extend their lives [4].
At the industrial commodity scale, the distribution of food may use an active cooling system, such as refrigerated trucks and cold storage facilities that can maintain the temperature continuously. However, the operation and maintenance of this system incur significant costs. Additionally, as vegetables and fruits are typically distributed in smaller quantities, using refrigerated trucks can lead to fluctuating market prices. An alternative solution is the use of cold storage containers, which offer a more cost-effective method for preserving perishables over short periods. To extend the preservation duration, incorporating a passive cooling system could be advantageous. This system facilitates thermal energy storage without the need for mechanical or electrical assistance, providing an efficient method to maintain temperature control [5].
PCM has shown potential as an effective passive cooling solution due to its ability to store a significant amount of latent heat within narrow temperature ranges during phase transitions, whether from solid to liquid or otherwise. This characteristic makes it particularly suitable for heat energy utilization in cold storage. During the charging process with an active cooling system, PCM releases energy through solidification, and during discharging when electricity is unavailable, it stores energy by melting. Additionally, PCMs have been proven to accelerate the freezing process, minimize temperature fluctuations, and substantially reduce thawing loss [6].
Water is acknowledged as a promising phase-change material (PCM) for cold storage applications owing to its low cost, high latent heat capacity, and superior thermal conductivity. Its abundance and non-toxic characteristics enhance its appeal from both economic and environmental standpoints. Nonetheless, optimal storage conditions for most fruits and vegetables are maintained between −2 °C and 5 °C to prevent spoilage and preserve freshness. Due to the temperature gradients inherent in heat transfer processes, water-based PCMs can lead to temperature inhomogeneities within the storage environment. Consequently, this may render water less effective as an ideal PCM for maintaining the requisite uniform temperature conditions in cold storage applications.
To address this limitation, extensive research has been conducted on eutectic salt solutions due to their significant latent heat of fusion [7,8,9]. Numerous studies have performed numerical and experimental investigations into phase-change materials (PCMs) in various configurations (sides, top, and bottom). However, this approach is not without its drawbacks. Salt solutions exhibit a critical disadvantage, supercooling, which can significantly reduce efficiency [10].
On the other hand, Huang and Nishinari [11] examined the impact of varying molecular weights of polyethylene glycol (PEG) on the enthalpy of fusion in eutectic mixtures. The researchers conducted experiments with a range of PEG concentrations to ascertain the optimal composition for thermal energy storage applications. Their findings showed that with 10 wt% PEG, the enthalpy of fusion of the mixture was nearly the same as that of the salt solution, ranging between 230 and 280 J/g, depending on the PEG’s molecular weight. Moreover, PEG is a non-toxic, odorless, neutral, lubricating, non-volatile, and non-irritating substance, which is classified as a food-grade material, making it suitable for applications in both medicinal and food products [12].
This research further enhances the configurations of PCM, demonstrating that the placement of PCM modules within a cooling system is not limited to traditional six-sided enclosures. By experimenting with various configurations and placement strategies, the study identified an optimal arrangement that maximizes cooling duration and efficiency. Additionally, while most existing research focuses on using salt solutions for low-temperature PCM applications, this study introduces a mixture of PEG6000 with water as an alternative PCM. This mixture potentially offers different thermal properties and performance benefits, expanding the range of materials available for cold-chain applications and improving the versatility and efficiency of thermal energy storage solutions.
2. Materials and Methods
2.1. Phase-Change Material (PCM)
This study employed potassium chloride (KCl) and polyethylene glycol 6000 (PEG6000) as primary materials for synthesizing phase-change materials (PCMs). KCl (CAS: 7447-40-7, 99% purity) was purchased from Rofa Laboratorium Centre, Bandung, Indonesia and used without further purification due to its high purity. PEG6000 (CAS: 25322-68-3), manufactured by PETRONAS Chemicals Group Berhad, Kuala Lumpur, Malaysia, has a melting point of 58 °C and an average molecular weight ranging from 5000 to 7000 g/mol, was selected for its appropriate thermal properties. These materials were subsequently dissolved in distilled water to form 10 wt% solutions of each substance. The resultant aqueous KCl and PEG6000 solutions were then encased in high-density polyethylene (HDPE) packs, each containing 200 g of the solution, sourced from Ice Pack Super, Yogyakarta, Indonesia, to evaluate their efficacy as PCMs within portable cold storage applications.
2.2. Experimental Setup
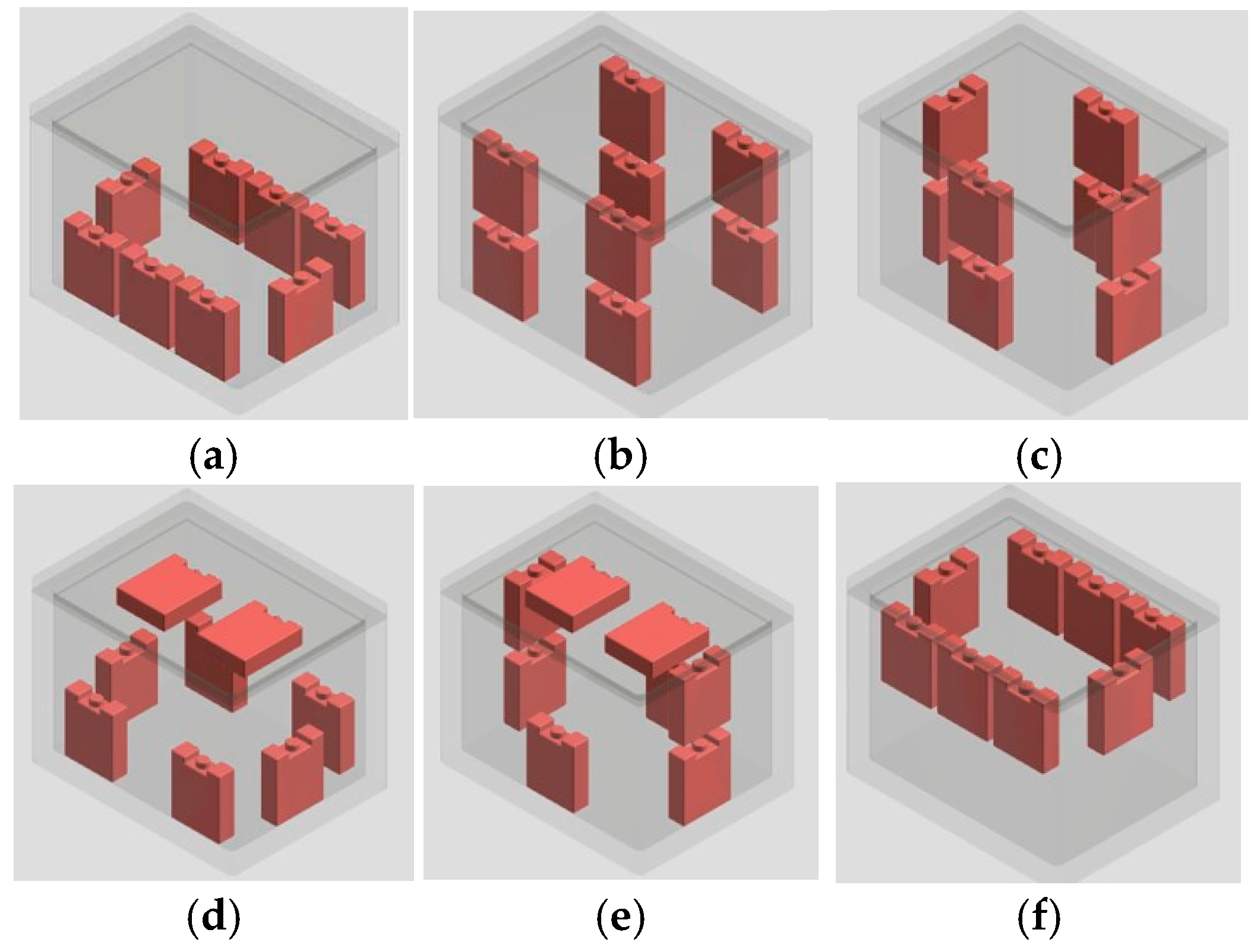
The experiment sought to explore the optimal PCM configurations by analyzing temperature inhomogeneity within cold storage, using a 10 wt% KCl solution. Additionally, the optimal configuration was employed to compare the performance of the 10 wt% KCl solution with that of a 10 wt% PEG6000 solution. The PCMs were arranged into six distinct configurations as illustrated in Figure 1 to maintain temperature stability and homogeneity, ensuring uniform cooling distribution within the given dimensions.
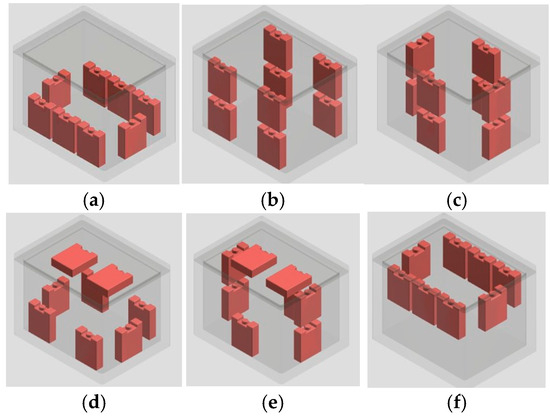
Figure 1.
PCM positions in the portable cold storage: (a) Layout 1, (b) Layout 2, (c) Layout 3, (d) Layout 4, (e) Layout 5, and (f) Layout 6.
In this experiment, a commercially used polystyrene container measuring 285 mm × 345 mm × 255 mm was employed due to its optimal balance of capacity and portability. These dimensions facilitate the single-handed transportation and efficient storage of products. To assess the efficacy of the phase-change materials (PCMs), eight PCM packs were strategically positioned along the inner walls of the container. The setup was then subjected to a preconditioning phase where it was stored in a freezer maintained at −25 °C for a duration of 24 h to ensure the PCM packs were fully solidified, maximizing their thermal energy storage potential. Following this, the container was removed from the freezer and placed in a room at ambient temperature to simulate real-world conditions without the presence of perishable goods, allowing for the evaluation of the PCM’s performance in maintaining the container’s internal temperature.
2.3. Temperature Measurements and Data Reduction
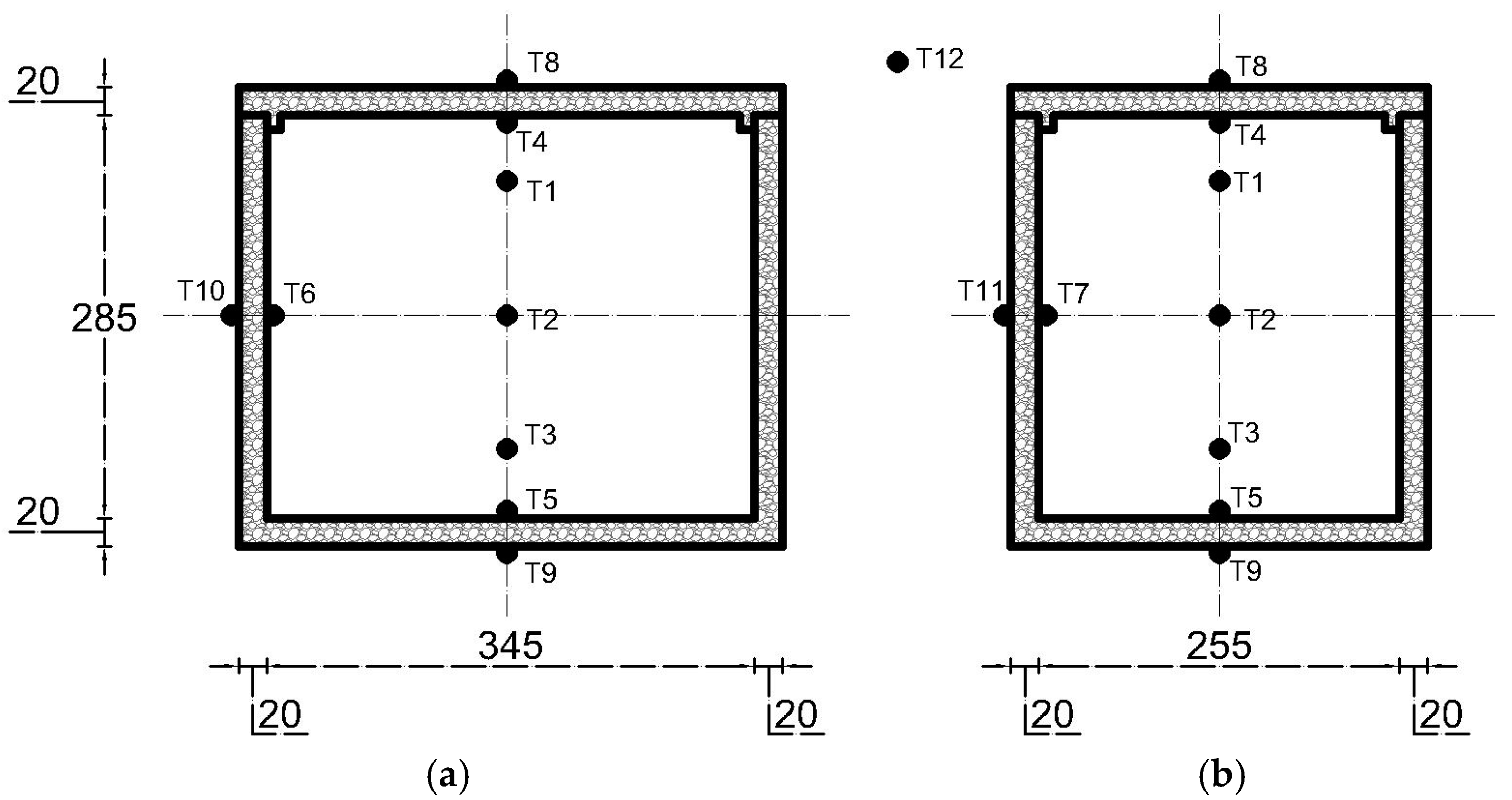
The experiment employed 12 thermocouples, as illustrated in Figure 2. In this study, temperature homogeneity was quantified by calculating the variation between the thermocouple readings using the standard deviation (σ). The standard deviation is defined as follows:
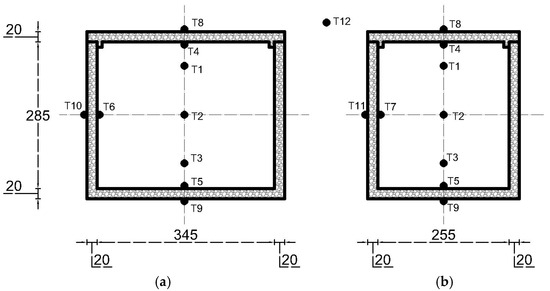
Figure 2.
Temperature measurements in portable cold storage from (a) front view and (b) side view.
Thermocouples were placed at different heights within the center of the cold storage to capture the temperatures, labeled as , , and . The average temperature across the storage is denoted by , and n refers to the total number of thermocouples used.
Furthermore, thermocouples were installed on the interior surfaces of four walls, represented as , , and , as well as on the exterior surfaces, marked as , , and . This setup assumes that the heat transfer load is uniform for both the front and back walls, as well as for the left and right walls. The heat rate from transmission load over a certain time period () is given by Equation (2).
Heat transfer will occur from the ambient conditions () into the cold storage, with the rate affected by the wall cross-sectional area of all six sides. In particular, indicates the area at the top, the area at the bottom, the area on the short sides, the area on the long sides. Other influential factors include the heat transfer distance () and the cold storage utilizes expanded polystyrene (EPS) for insulation, recognized for its thermal conductivity (k) of 0.033 W/m2K [13].
3. Results
3.1. Temperature Uniformity and Performance in Different Configurations
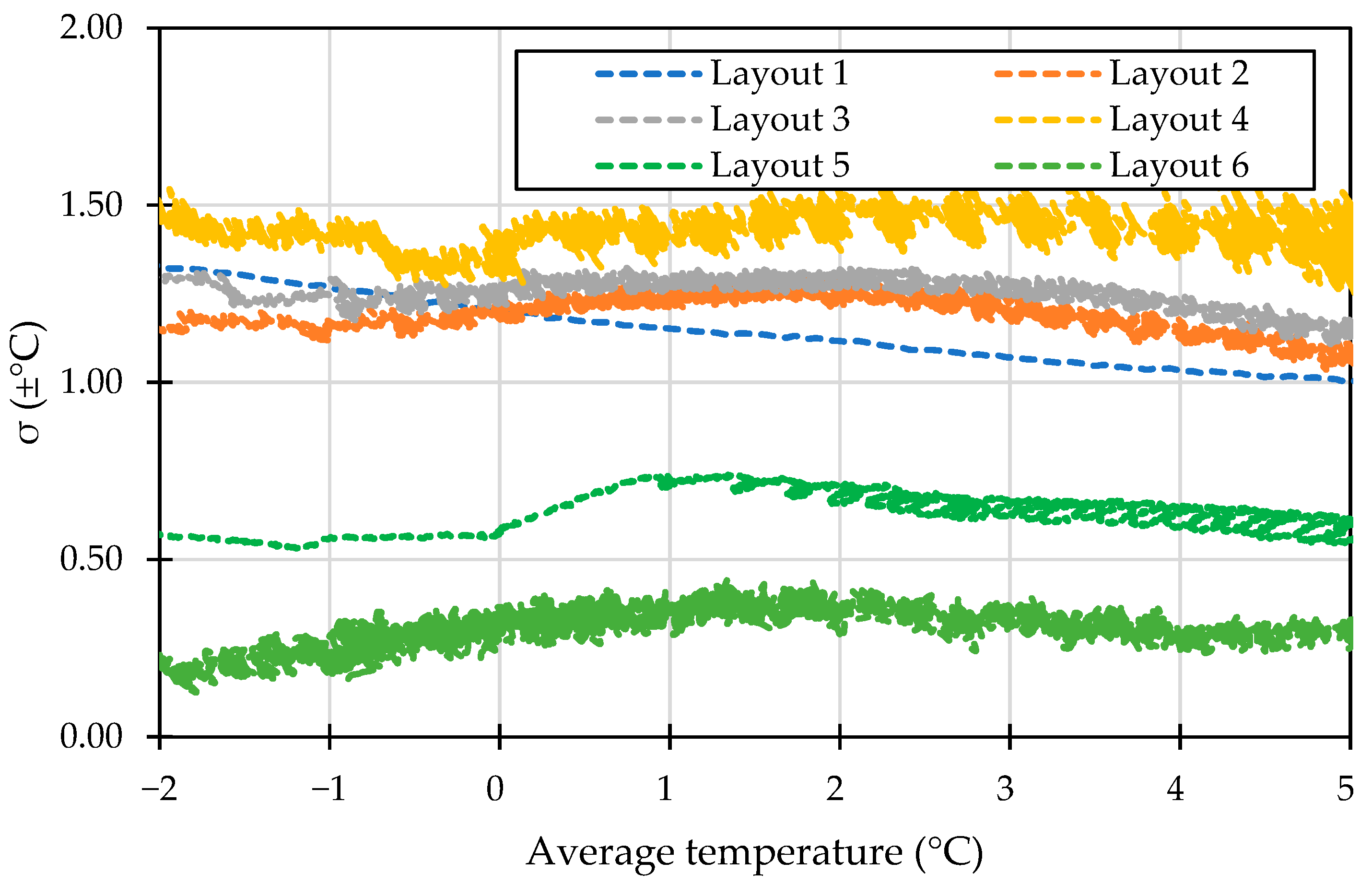
This section examines the impact of PCM configurations on cooling performance. Figure 3 presents a comparison of the standard deviation in temperature distribution across six PCM layouts at the target temperature, representing the temperature inhomogeneity across the cold storage. Layouts 1, 2, 3, and 4 showed a standard deviation above 1 °C, with Layout 4 having the highest temperature disparity, reaching a maximum standard deviation of ±1.57 °C within the optimal temperature range for perishable foods. In contrast, Layouts 5 and 6 displayed better temperature uniformity, with a standard deviation below 1 °C. However, Layout 6 demonstrated the best uniformity, with an average standard deviation of ±0.27 °C. This level of uniformity is crucial for transporting fruits and vegetables that require narrow temperature ranges to ensure that the entire storage remains within safe limits.
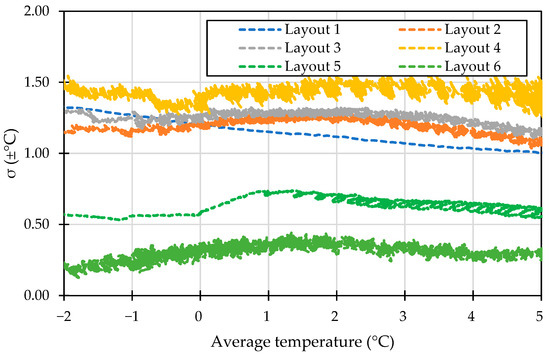
Figure 3.
The standard deviation of storage temperature in different configurations.
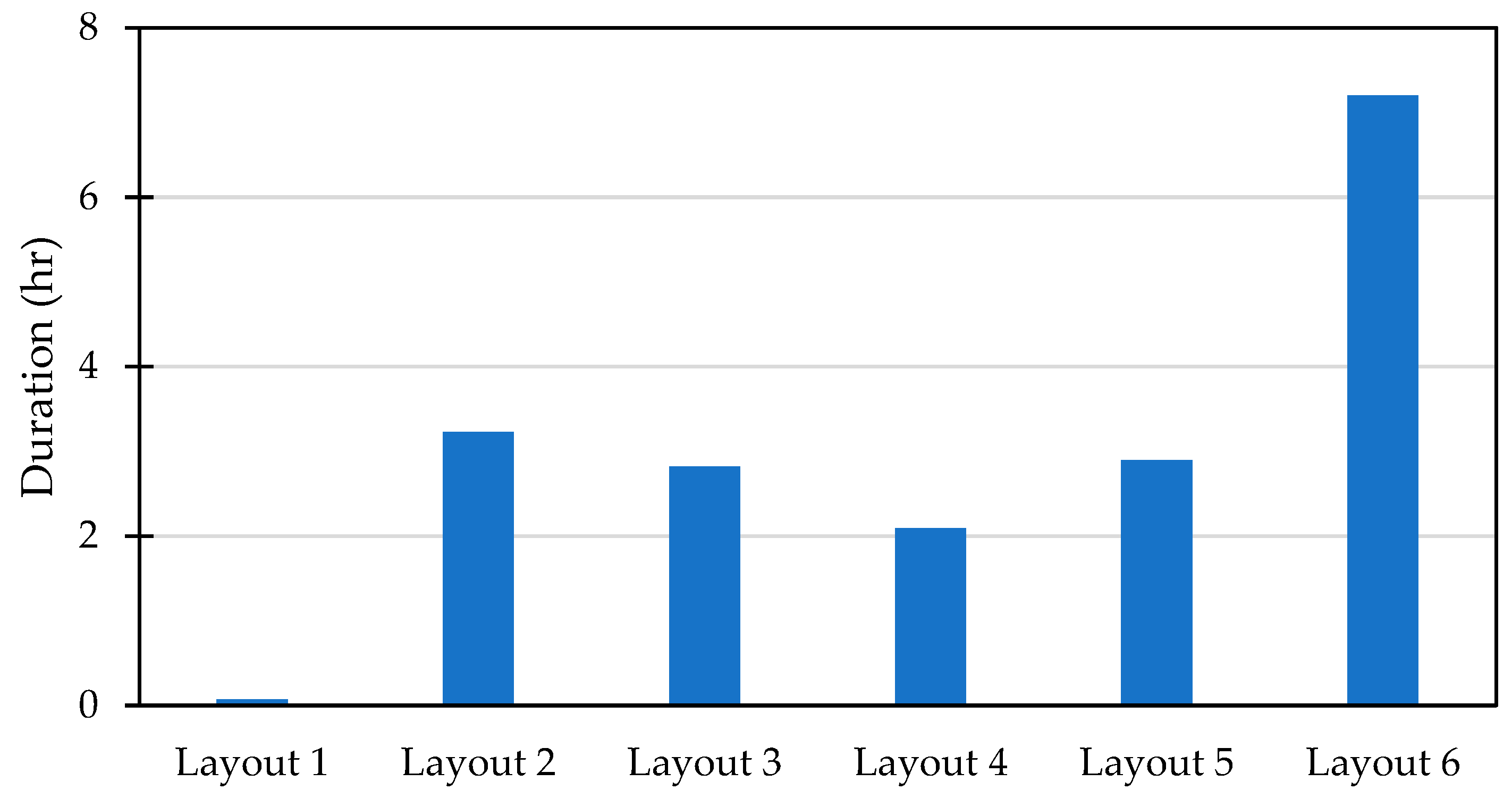
Despite Layout 5 having a lower temperature disparity, its performance was nearly the same as Layout 3, with storage durations of 2.89 h and 2.82 h, respectively. However, both were less effective compared to Layout 2, which managed to maintain the target temperature for 3.23 h, as shown in Figure 4. While Layout 5 outperformed Layout 4 slightly, it still fell short in overall efficiency. On the other hand, Layout 1 proved to be the least effective configuration, offering the shortest storage duration and thus making it unsuitable for the cooling requirements. In contrast, Layout 6 stood out as the most optimal configuration, providing a significantly longer storage duration of up to 7.2 h, making it the best choice for maximizing cooling efficiency and maintaining the desired temperature for extended periods.
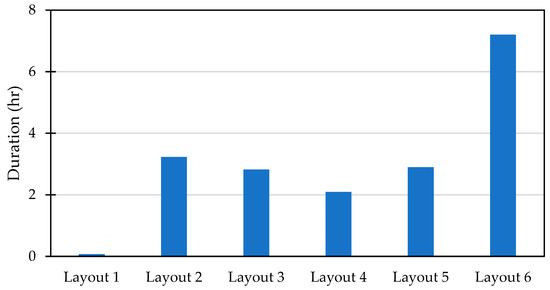
Figure 4.
The duration of 3 thermocouples in the middle at temperatures from −2 to 5 °C.
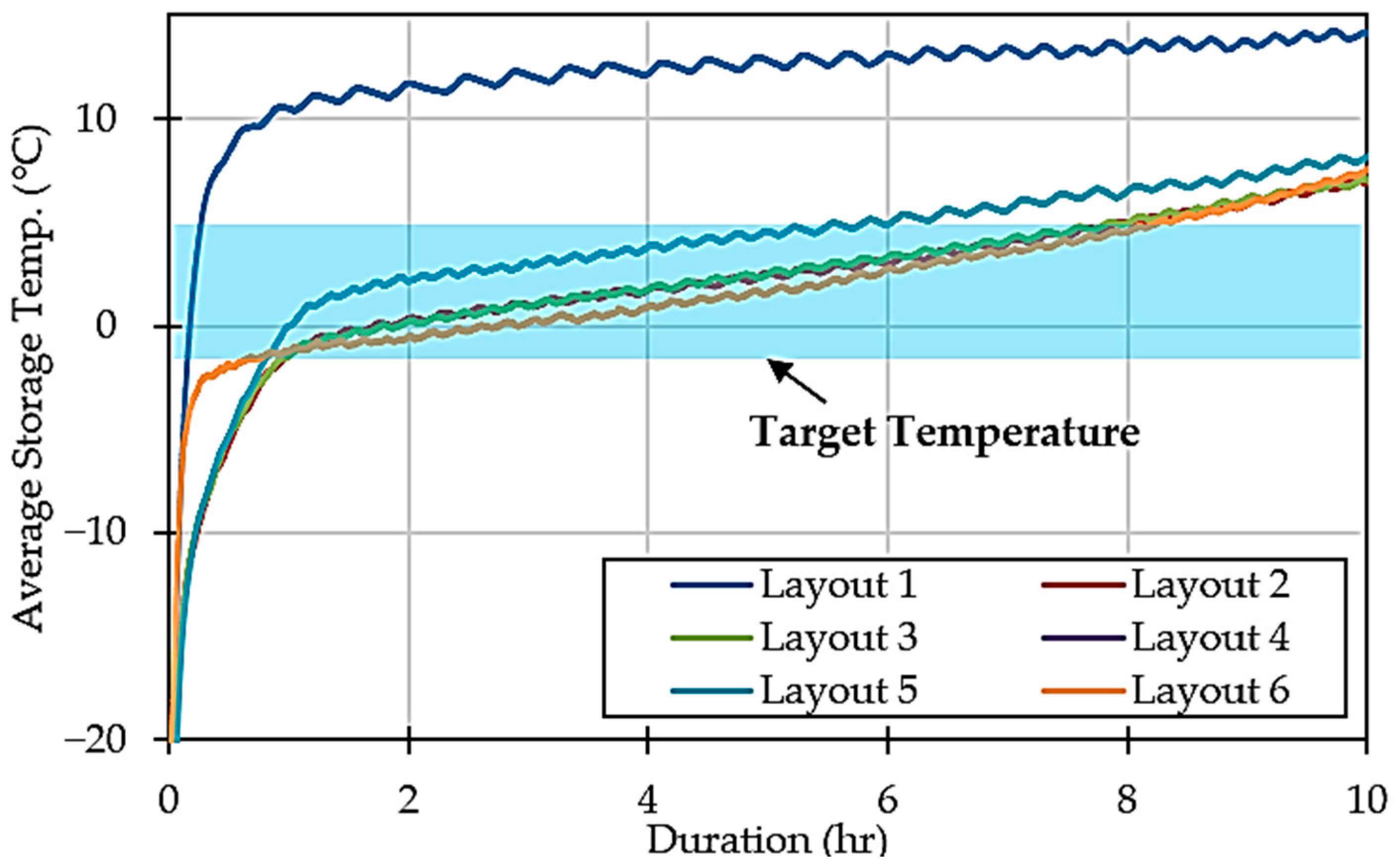
The analysis reveals that Layout 1 is incompatible with storing perishable food at the target temperature. This is also supported by Figure 5, which shows a significant rise in average storage temperature, eventually surpassing 10 °C. Furthermore, Layout 5 also struggled to maintain lower temperatures, with the average temperature rise beginning to slow at around 0.95 °C, whereas other configurations, like Layouts 2, 3, and 4, showed a decrease of around −0.97 °C. Layout 6 performed particularly well, demonstrating an ability to effectively counteract temperature increases, achieving a reduction of −2.78 °C.
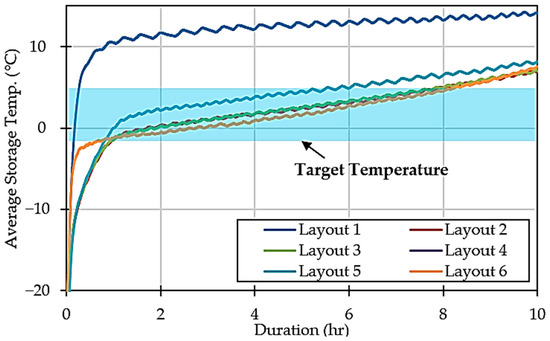
Figure 5.
Average storage temperature over time in different configurations.
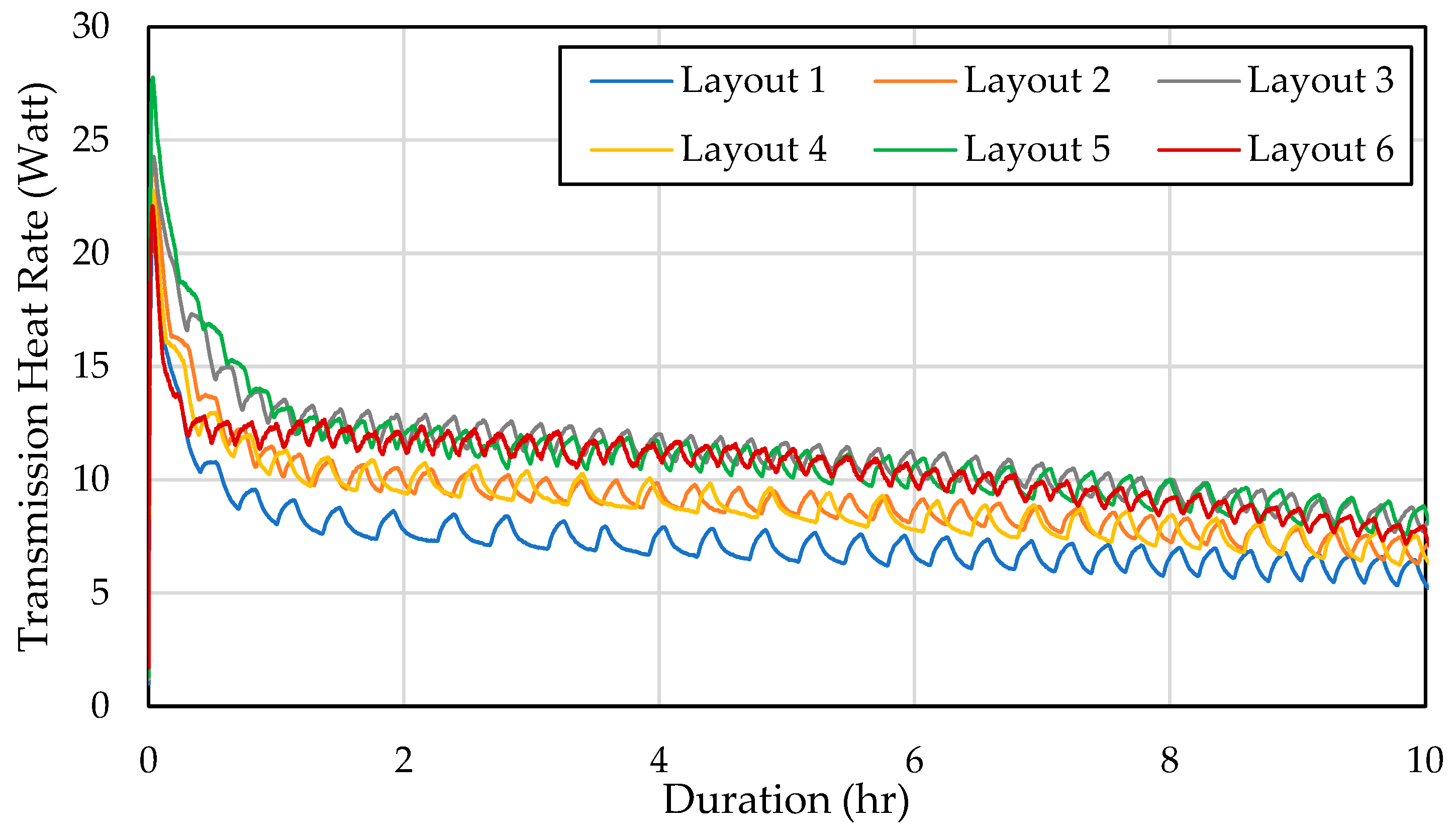
Furthermore, Figure 6 highlights that Layout 5 experienced significant heat loss through the walls due to the PCM being scattered, causing all walls to cool more than in other configurations. Subsequently, Layout 6 also encountered a high-transmission heat load, though this was a result of its storage temperature remaining consistently lower than the other layouts over time. In contrast, Layout 1 exhibited the lowest-transmission heat load of all configurations. This was attributed to its higher storage temperature, which minimized the temperature differential between the interior and exterior walls, thereby reducing heat transfer.
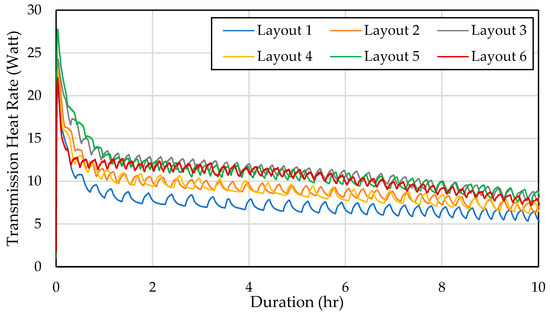
Figure 6.
Heat rate through the wall over time in different configurations.
Overall, Layout 6 demonstrated excellent temperature uniformity and maintained the lowest storage temperature. This performance was achieved because the PCM cooled the air at the top of the storage unit. As the temperature at the top decreased, the density of the air at the top increased, causing greater natural convection.
3.2. Aqueous PEG6000 Compared to Aqueous KCl
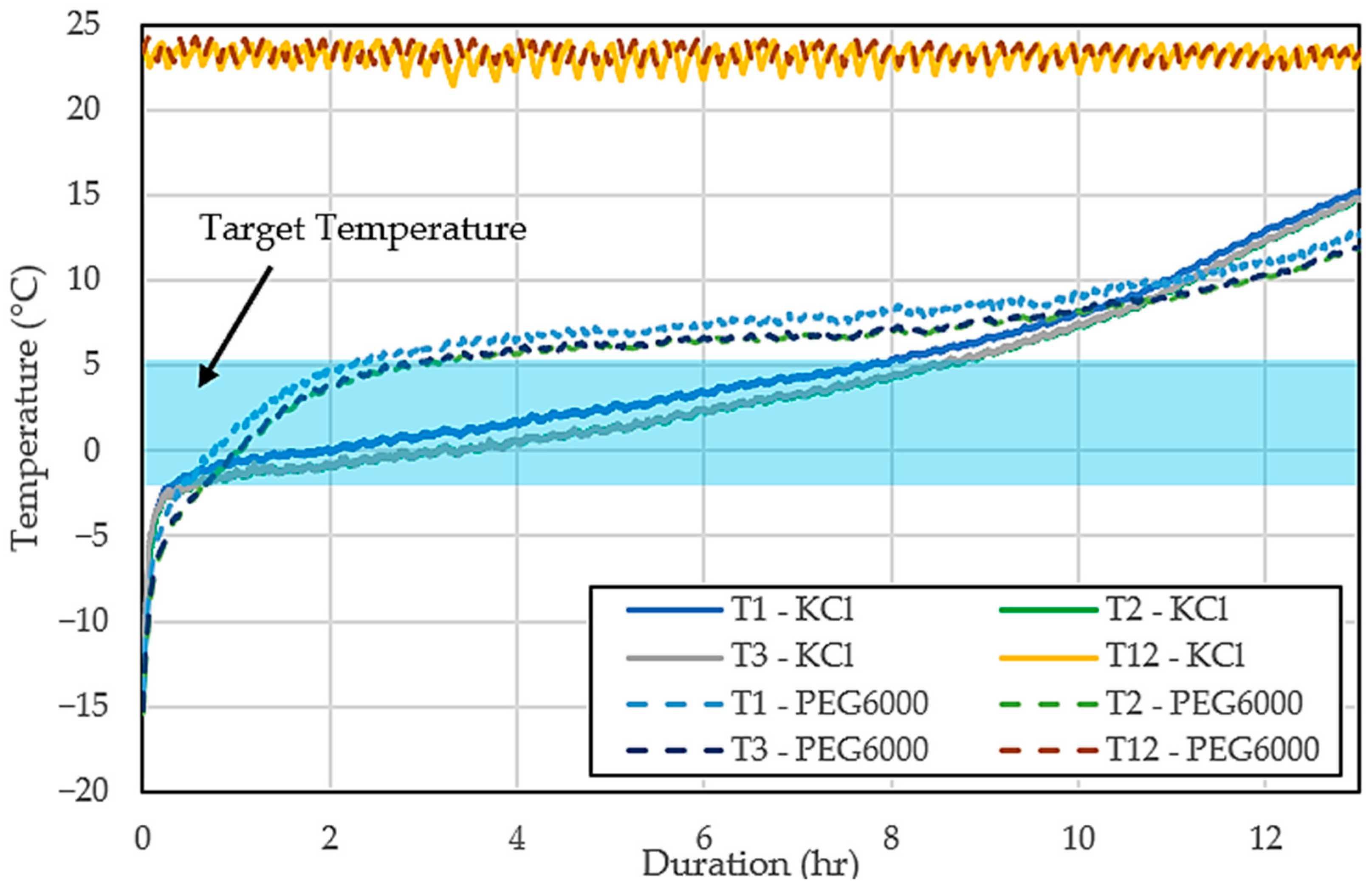
Figure 7 displays the temperature profile of the cold storage and the ambient conditions over time while comparing the performance of two different PCMs, the 10 wt% aqueous KCl solution and the 10 wt% aqueous PEG6000 solution, both under relatively constant ambient temperatures. At first, the temperature inside the cold storage rises quickly as it approaches the target temperature. As time passes, the cold storage containing the KCl solution stabilizes, maintaining a temperature close to −2.78 °C, which successfully keeps the storage within the desired range. In contrast, the temperature in the storage using the PEG6000 solution continues to rise steadily and eventually exceeds the target temperature. After this initial increase, the PEG6000 solution stabilizes at a higher temperature of around 4 °C. This result shows that while the PEG6000 solution is capable of providing cooling, it maintains temperatures slightly above the desired range.
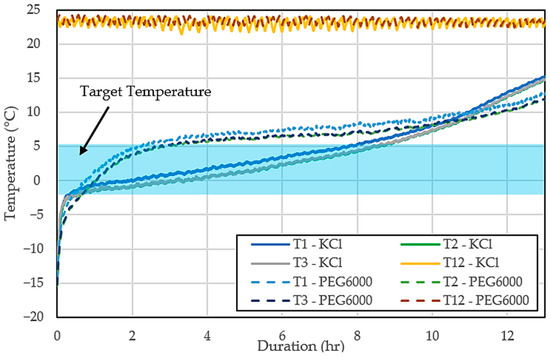
Figure 7.
Cold storage and ambient temperatures with 10 wt% aqueous KCl solution and 10 wt% aqueous PEG6000 as PCM.
4. Conclusions and Future Works
Based on the experimental results, it can be concluded that the optimal configuration for the portable cold storage was achieved by positioning the PCM packs at the upper side of the unit. This arrangement provided the highest level of temperature uniformity, with a standard deviation of approximately ±0.27 °C, indicating minimal temperature homogeneity. Additionally, this setup optimized the heat transfer from the PCM, effectively maintaining stable temperatures within the storage space, and making it highly suitable for cold storage applications.
With the PCM packs positioned on the upper side, the portable cold storage was able to maintain the desired temperature for up to 7.2 h when utilizing a eutectic PCM consisting of a 10% aqueous KCl solution. In comparison, the 10% aqueous PEG6000 solution also demonstrated the ability to sustain temperatures, but the temperatures remained slightly higher than those maintained by the aqueous KCl solution. The PEG6000 solution, although performing at slightly elevated temperatures, could still be advantageous in situations where a marginally higher temperature range is acceptable.
Author Contributions
Conceptualization, H.M.A. and I.P.; Data curation, I.M., H.M.A. and Y.Y.A.; Formal analysis, I.M. and H.M.A.; Investigation, I.M., H.M.A. and Y.Y.A.; Methodology, I.M., H.M.A. and I.P.; Validation, I.M. and H.M.A.; Visualization, I.M., H.M.A. and Y.Y.A.; Writing—original draft, I.M. and H.M.A.; Supervision, H.M.A. and I.P.; Funding acquisition, H.M.A.; Project administration, H.M.A.; Writing—review & editing, H.M.A., I.P. and Y.Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known conflicts of interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Leungtongkum, T.; Flick, D.; Hoang, H.M.; Steven, D.; Delahaye, A.; Laguerre, O. Insulated box and refrigerated equipment with pcm for food preservation: State of the art. J. Food Eng. 2022, 317, 110874. [Google Scholar] [CrossRef]
- Bai, L.; Liu, M.; Sun, Y. Overview of Food Preservation and Traceability Technology in the Smart Cold Chain System. Foods 2023, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Wang, X.; Rahbari, A.; Hangi, M. Optimisation of a portable phase-change material (PCM) storage system for emerging cold-chain delivery applications. J. Energy Storage 2022, 52, 104855. [Google Scholar] [CrossRef]
- Lad, P.; Kumar, R.; Saxena, R.; Patel, J. Numerical investigation of phase change material assisted indirect solar dryer for food quality preservation. Int. J. Thermofluids 2023, 18, 100305. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Zhao, Y.; Torres, J.F.; Wang, X. PCM-based ceiling panels for passive cooling in buildings: A CFD modelling. Energy Build. 2023, 285, 112898. [Google Scholar] [CrossRef]
- Hakim, A.R.; Fauzi, A.; Pranoto, I. Phase change material-assisted freezing of tuna: Impact on freezing process and thawing loss. J. Food Process Eng. 2022, 45, e14011. [Google Scholar] [CrossRef]
- Kozak, Y.; Farid, M.; Ziskind, G. Experimental and comprehensive theoretical study of cold storage packages containing PCM. Appl. Therm. Eng. 2017, 115, 899–912. [Google Scholar] [CrossRef]
- Zarajabad, O.G.; Ahmadi, R. Numerical investigation of different PCM volume on cold thermal energy storage system. J. Energy Storage 2018, 17, 515–524. [Google Scholar] [CrossRef]
- Sukoco, R.K.; Indartono, Y.S.; Mujahidin, D. Eutectic potassium chloride (KCl) solution as phase change material (PCM) for fish cold storage. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1109, 012062. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, R.; Li, J. High latent heat phase change materials (PCMs) with low melting temperature for thermal management and storage of electronic devices and power batteries: Critical review. Renew. Sustain. Energy Rev. 2022, 168, 112783. [Google Scholar] [CrossRef]
- Huang, L.; Nishinari, K. Interaction between poly(ethylene glycol) and water as studied by differential scanning calorimetry. J. Polym. Sci. B Polym. Phys. 2001, 39, 496–506. [Google Scholar] [CrossRef]
- Moo-Young, M. Comprehensive Biotechnology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yucel, K.T.; Basyigit, C.; Ozel, C. Thermal Insulation Properties of Expanded Polystyrene as Construction and Insulating Materials. 2003. Available online: https://www.researchgate.net/publication/237669763 (accessed on 18 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).