Flocculation of Sulfide Minerals with a Mixture of Potassium Xanthate Butyl and Water-Soluble Polymer †
Abstract
1. Introduction
2. Methodology
- (a)
- the formation of “bridges” between mineral particles due to the hydrogen bond of polymer molecules with the surface;
- (b)
- electrostatic interaction between the charges of the ionized polymer and the particle;
- (c)
- in some cases, with a reaction between the functional groups of polymers and metal ions on the surface of the mineral.
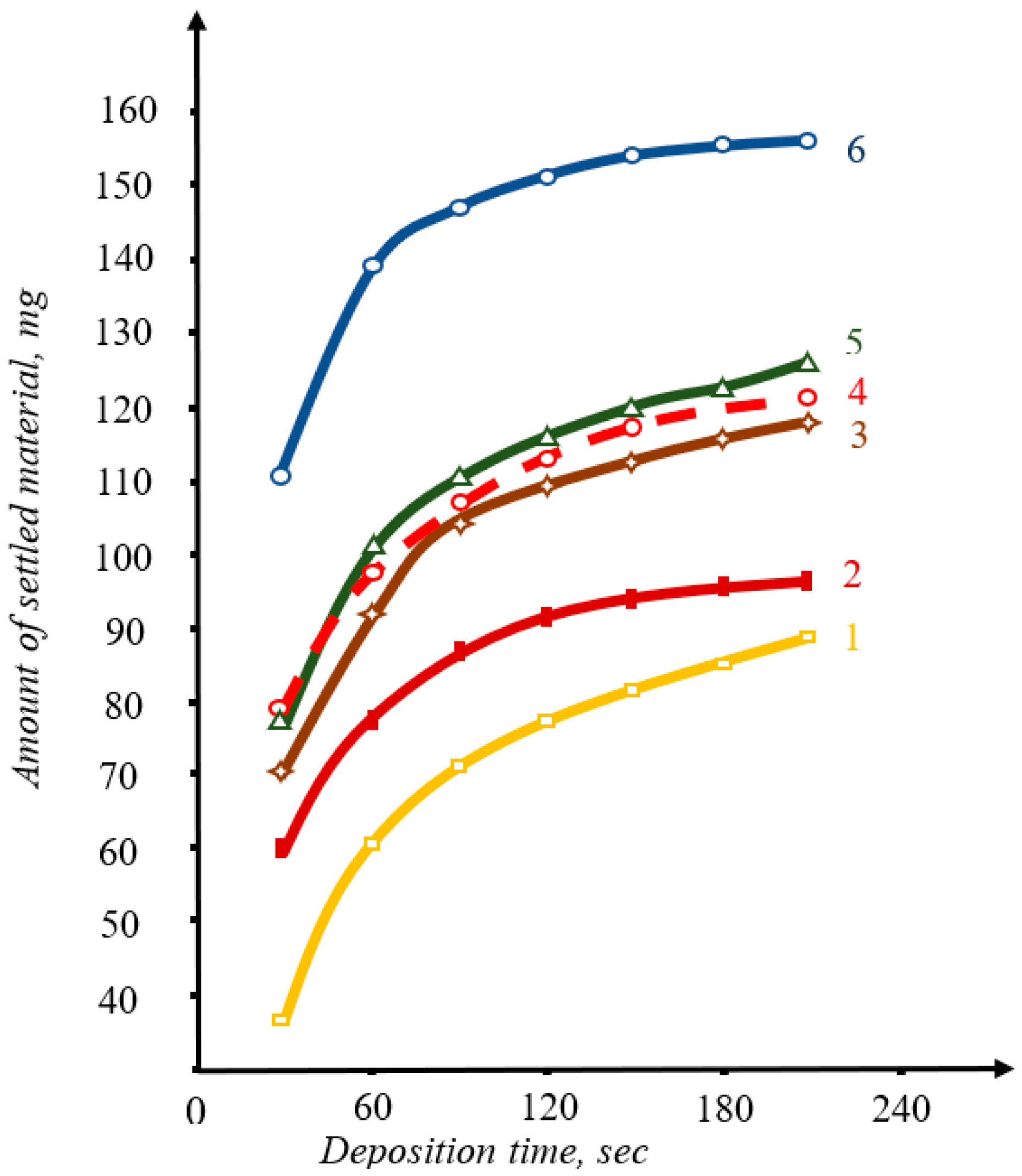
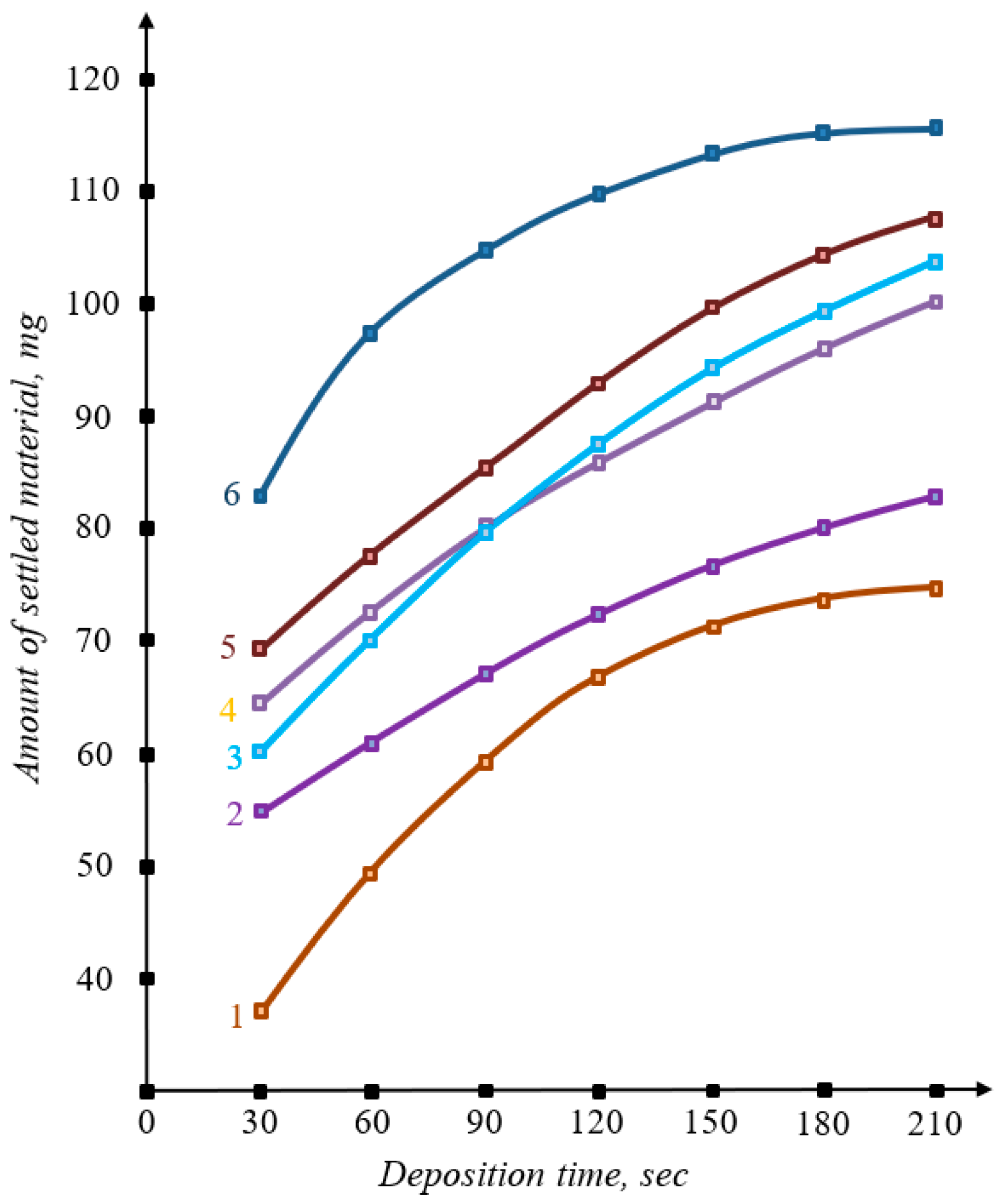
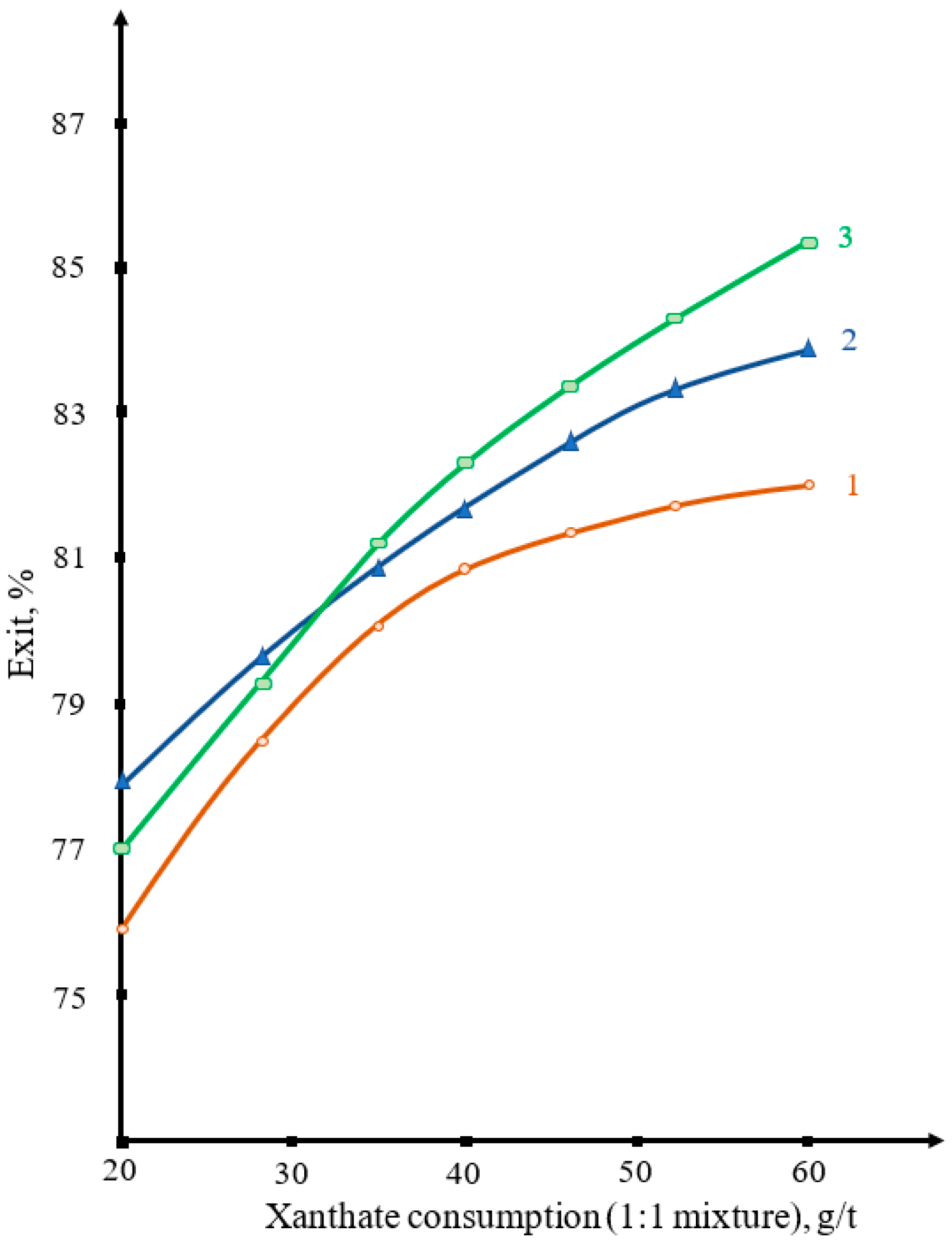
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminzhanova, S.I.; Mishareva, M.E. Research on the technology of using water-soluble polyelectrolytes as flotation reagents. Sci. Prog. 2022, 3, 369–377. [Google Scholar]
- Mishareva, M.E. Study of the use of water-soluble polymers in flotation beneficiation of ores. Sci. Prog. 2021, 2, 879–887. [Google Scholar]
- Menkovsky, M.A. On a method for studying the kinetics of sedimentation of coarse sludge. Min. J. 1966, 2, 73. [Google Scholar]
- Abramov, A. Innovative technologies for environmental protection during processing and enrichment of minerals. Subsoil Use XXI Century 2009, 6, 61–67. [Google Scholar]
- Berger, G.S.; Lerner, V.N. A new device for determining the flocculation of mineral grains in aqueous suspensions. In Colloquium on the Theory of Flotation; Alma-Ata, “Science”: Moscow, Russia, 1970; pp. 254–256. [Google Scholar]
- Bragin, V.I. Development of Physical and Chemical Methods for Preparing Mineral Raw Materials for Enrichment: Dis; Siberian Federal University: Krasnoyarsk, Russia, 2014. [Google Scholar]
- Wright James, T.; White Karl, R.; Gabrielson, K.; Hines John, B.; Arthur Lisa, M.; Kausin Michael, J.; Georgia-Pacific Chemicals, L.C. Modified Amino Aldehyde Resins and Their Application in Separation Processes. Russia Patent RU2432998C2, 10 November 2011. [Google Scholar]
- Komogortsev, B.V.; Varenichev, A.A. Improving technologies for flotation enrichment of finely dispersed sulfide gold ores. Min. Inf. Anal. Bull. (Sci. Tech. J.) 2018, 10, 180–190. [Google Scholar]
- Chanturia, V.A. New reagents for the extraction of noble metals from difficult-to-process ores and products. Phys.-Tech. Probl. Miner. Dev. 2010, 1, 78. [Google Scholar]
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Berdiyarov, B.T. Technological processes of receiving metals in the conditions of moderate temperatures. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 1826–1828. [Google Scholar] [CrossRef]
- Kholikulov, D.B. Pilot tests of processing technologies of process solutions of copper production by ozonation. Mater. Today Proc. 2021, 45, 4987–4992. [Google Scholar] [CrossRef]
- Somasundaran, P.; Nagaraj, D.R. Chemistry and applications of chelating agents in flotation and flocculation. Reag. Miner. Ind. 1984, 96, 209–219. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Pugh, R.J. Macromolecular organic depressants in sulfide flotation-a review, 1. Principles, types and applications. Int. J. Miner. Process. 1989, 25, 101–130. [Google Scholar] [CrossRef]
- Umarova, I.K.; Matkarimov, S.T.; Makhmarezhabov, D.B. Development of flotation technology for gold-bearing ores of the Amantaytau deposit. Obogashchenie Rud 2020, 2, 29–33. [Google Scholar] [CrossRef]
- Uzoni, L.; Rinelli, G. Selective properties of flocculants and the possibilities of their use in the flotation of fine ores. In Proceedings of the VII International Congress on Mineral Processing, Moscow, Russia, 20–24 September 1968; p. 72. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhmarezhabov, D.; Matkarimov, S.; Yavkochiva, D.; Yuldasheva, N. Flocculation of Sulfide Minerals with a Mixture of Potassium Xanthate Butyl and Water-Soluble Polymer. Eng. Proc. 2024, 63, 27. https://doi.org/10.3390/engproc2024063027
Makhmarezhabov D, Matkarimov S, Yavkochiva D, Yuldasheva N. Flocculation of Sulfide Minerals with a Mixture of Potassium Xanthate Butyl and Water-Soluble Polymer. Engineering Proceedings. 2024; 63(1):27. https://doi.org/10.3390/engproc2024063027
Chicago/Turabian StyleMakhmarezhabov, Dilmurod, Sokhibjon Matkarimov, Dilfuza Yavkochiva, and Nasiba Yuldasheva. 2024. "Flocculation of Sulfide Minerals with a Mixture of Potassium Xanthate Butyl and Water-Soluble Polymer" Engineering Proceedings 63, no. 1: 27. https://doi.org/10.3390/engproc2024063027
APA StyleMakhmarezhabov, D., Matkarimov, S., Yavkochiva, D., & Yuldasheva, N. (2024). Flocculation of Sulfide Minerals with a Mixture of Potassium Xanthate Butyl and Water-Soluble Polymer. Engineering Proceedings, 63(1), 27. https://doi.org/10.3390/engproc2024063027






