Abstract
The main energy used today is fossil energy which is decreasing and cannot be replaced. Meanwhile, the Indonesian people themselves are facing a problem as well as a great opportunity, namely, plastic waste and the potential of use rice husks, respectively. This study aimed to determine the most effective flame and most effective temperature for combustion in rice husk gasification, and to determine the temperature in the distillation reactor, and then to determine the mass of oil produced from the pyrolysis process and determine the quality of PP (polypropylene) plastic pyrolysis oil. The method used was direct experiment. The test was carried out using two furnaces and was carried out in parallel six times. The results showed that the gasification of rice husk briquettes could be a source of combustion in the pyrolysis process. The time used in the pyrolysis process was 260 min while the average flame duration in the furnace over six test was 43.33 min. The average flame temperature over the six periods of combustion was 733.89 °C. Additionally, the average temperature at the bottom of the reactor was 593.564 °C, and the average temperature in the reactor chamber was 143.319 °C. This process was categorized as low-temperature pyrolysis. The mass produced from 3000 g of shredded PP (polypropylene) plastic waste produces 1.29 L of fuel oil. It is of note that the quality of plastic pyrolysis oil is not greatly different from kerosene, with a thermal efficiency of 38.60%.
1. Introduction
It cannot be denied that human dependence on energy is great. Almost all sectors of life require energy. The main source of energy used is fossil energy. Imperceptibly, the world fossil fuels are running low; meanwhile, their consumption remains high across the world, including in Indonesia. Additionally, Indonesia has a problem, albeit one with great potential. This problem is plastic waste. The generation of plastic waste in Indonesia is very high. The activities of everyday human life can never be separated from the use of plastic. It can be said that it is difficult to separate plastics from human life. The use of plastic is considered to provide convenience and practicality in meeting primary, secondary and tertiary needs.
The accumulation of garbage in final disposal sites (TPAs) is already very large. The production of plastic materials in Indonesia reached 7.23 million tons in 2018, with a growth rate of around 5% per year. Polymer waste has the potential to be used as a cheap source of chemicals and energy. Polymer incineration creates a serious air pollution problem because it releases harmful gases such as dioxins, hydrogen chloride, airborne particles, and carbon dioxide. Also, plastics are not able to be disposed of in landfills (TPAs) because of their high cost and poor biodegradability [1].
Of course, such a large amount of production, also produces large amounts of waste. Consequently, the growth in waste production per year can reach 5%. This means that production in 2019 reached 7.59 million tons, and 7.96 million tons in 2020, and in 2021 plastic waste production reached 8.35 million tons. Moreover, plastic waste also increasingly circulates in the surrounding environment. Imagine if, in the future, the amount of plastic production were to increase. In that scenario plastic waste would also increase. One way to reduce this plastic waste is by recycling or converting it into a new energy source.
Aside from these problems, Indonesia has great potential in the form of abundant biomass. This can be utilized by using gasification technology, where gasification technology itself is the process of converting solid objects in the form of biomass or coal into gas. Many studies have explored the development of biomass using gasification technology as a means of finding new and renewable energy. In practice, combustion using gasification with biomass is easier, cheaper, and more practical and can even have the potential to produce higher heating power than the flames from the LPG gas that is on the market, and biomass also has better combustion potential.
At this time, efforts are being made to develop a method for converting this waste into alternative energy sources. Apart from being able to overcome the problems of the energy crisis that is being addressed, and will be addressed in the future, converting plastic waste into fuel oil can create more business opportunities in the future.
1.1. Literature Review
Several previous research results show that many forms of plastic waste are converted into fuel oil. In addition, research on rice husks to be used as fuel is also being widely studied. The studies conducted include the type of plastic used in converting plastic waste into fuel oil, discussions about tools for converting plastic into oil, the use of gasification for igniting rice husks, and also the process of making briquettes from various materials and binders.
Currently, innovations in the conversion of plastic into fuel oil and the optimal utilization of rice husks are continuously being developed. Several studies related to the development of plastic waste into oil and the use of rice husks include [2] LDPE (low-density polyethylene) plastic waste, which is processed as an alternative fuel. The results showed that the volume of plastics that can be processed is currently around 4%. In [3], the study investigated pyrolysis, which is the chemical decomposition of organic matter through a heating process with no, or a limited oxygen or other chemical reagents, where the raw material undergoes a chemical structure breakdown into a gas phase. After the oil is distilled, the analysis includes the heating calorific value of combustion, flash point, ash content, moisture content, and composition analysis. In [4], it is recorded that pyrolysis process is chosen by most researchers among other thermal treatment technologies primarily because of its potential to convert most of the energy from plastic waste into valuable liquid oil, gas, and charcoal. In [5], there is a study comparing of the first type of plastic waste type 1, polyethylene terephthalate (PET or PETE or polyester), and type 2, high-density polyethylene (HDPE), with a ratio of 1:1 plastic waste. In [6], the study investigates turning plastic into fuel, and solving two problems: one is the huge ocean of plastic, and the other is the lack of fuel. The study [1] concern the analysis of solid plastic waste recycling techniques. Recycling can be divided into four categories: primary, secondary, tertiary, and quaternary. In [7], there is research on the use rice husks and their effectiveness as an alternative renewable energy sources, for household needs, using rice husk stoves to increase efficiency. In [8], the study provides a basic overview of gasification, gasification mechanisms, types of gasification, and the different characteristics of biomass. In [9], research is conducted to determine the difference between continuous and intermittent rice husk gasification systems by installing a modified burner. The difference can be measured based on the effectiveness of the run time and the average temperatures produced by the two systems. In [10], there is a discussion of the process of manufacturing biomass briquettes from Cerbera mango leaf waste can be used as a sustainable energy source with an optimal composition of 90% leaf waste and 10% tapioca.
The important factors in plastics are the various types of plastic, their uses, and the thermal properties of the plastic material or their heat properties. Consequently, as explained by [11] in his research, Table 1 and Table 2 show that knowledge of various types plastic and the thermal properties of plastics is very important in the process of making or recycling plastics. The plastic types and their uses can be seen in Table 1.

Table 1.
Types of plastics, codes, and their use [11].

Table 2.
Transition temperature and plastic melting temperature data [11].
The Important thermal properties are the melting point (Tm), transition temperature (Tg), and decomposition temperature. The transition temperature is the temperature at which the plastic undergoes structural relaxation with the result that it changes from being rigid to being more flexible. The melting temperature is the temperature at which the plastic begins to soften and turn into a liquid. The decomposition temperature is a limitation of the liquefaction process. If the temperature is raised above the melting temperature, the plastic will flow easily and the structure will decompose. The thermal property data in the plastic recycling process can be seen in the Table 2.
Pyrolysis is the process of heating a substance, e.g., coconut shell, in the absence of oxygen so that there is a decomposition of the components that make up the substance. Another description of pyrolysis is the irregular decomposition of organic materials caused by heating without contact with outside air. It has also been explained that this pyrolysis process has two stages, namely the low-temperature pyrolysis stage (0 °C–200 °C) and the high-temperature stage (above 200 °C) [12].
According to [7], gasification is the process of converting solid fuels (e.g., biomass, coal) into gas products using air/O2/H2O/CO2, or a mixture of them with a reactant ratio between 20 and 70% of their stochiometric requirements. The product gas is known as producer gas, which consists of combustible gases (CO, H2, and CH4) and non-combustible gases (CO2 and N2). These gases can be used as an energy source. Apart from these gases, producer gas also contains tar and other contaminants.
From the above explanation, we learn that the gasification process is the process of forming gas fuel (CO, H2, and methane (CH4)) from the results of chemical reactions sourced from biomass. Biomass can be in the form of rice husks, sawdust, coal, and agricultural, and plantation waste. Moreover, we can also conclude that gasification is a process of converting solids into gasses at a high temperature. Additionally, the gasification process is good because the results of the combustion do not cause gases that are harmful to humans. Meanwhile, in this study, the modified gasification stove was used as the main means of causing combustion.
The water boiling test is a method that is often used as a parameter to test combustion such as gasification or oil quality test. In this study, the water boiling test was used to test the quality of the PP plastic waste pyrolysis results by burning rice husk briquettes to achieve gasification. The water boiling test was carried out by heating water with a mass of 1 kg. Then, the water temperature was observed until it reached 100 °C. Next, after reaching 100 °C, we continued to heat the water until the fuel ran out in order to observe how much of the mass of the water was lost.
In his book, the author of [13] describes several parameters in testing combustion.
1.2. Sensible Heat
Sensible heat is the amount of heat energy required to raise the temperature of the water. This is measured before and after the water reaches the boiling temperature.
where, is mas of water (kg), is water specific heat (Kcal/kg °C), is the temperature of water at boiling point (°C), and is room temperature (°C).
1.3. Latent Heat
Latent heat is the amount of heat energy used in evaporating water.
where is the weight of water evaporated (kg), and is the latent heat of the water (540 Kcal/kg).
1.4. Heat Energy Input
Heat energy input is the amount of heat energy available in the fuel. This is computed using the formula,
where is the weight of the fuel (kg) and is the heating value of the fuel (Kcal/kg).
1.5. Thermal Efficiency
The thermal efficiency is the ratio of the energy used in boiling and evaporating water to the heat energy available in the fuel.
where is sensible heat (Kcal), is latent heat (Kcal), is the heating value of the fuel (Kcal/kg), and is weight of the fuel (kg)
Based on previous research [14], explained that the PP plastic pyrolysis oil with a processing time of 3 h was 11,670 cal/gr, the processing time for 2 h was 10,062 cal/gr and the processing time for 1 h was 10,295 cal/gr. Meanwhile, the comparison of kerosene has a calorific value of 10,478.95 cal/gr according to the data archive of the Ministry of Energy and Mineral Resources of the Republic of Indonesia. Therefore, the researchers used the data as a theoretical reference. While, according to research results [15] the density of fuel kerosene is 0.78 gr/mL and the density of plastic PP pyrolysis oil is 0.8 gr/mL.
2. Research Methodology
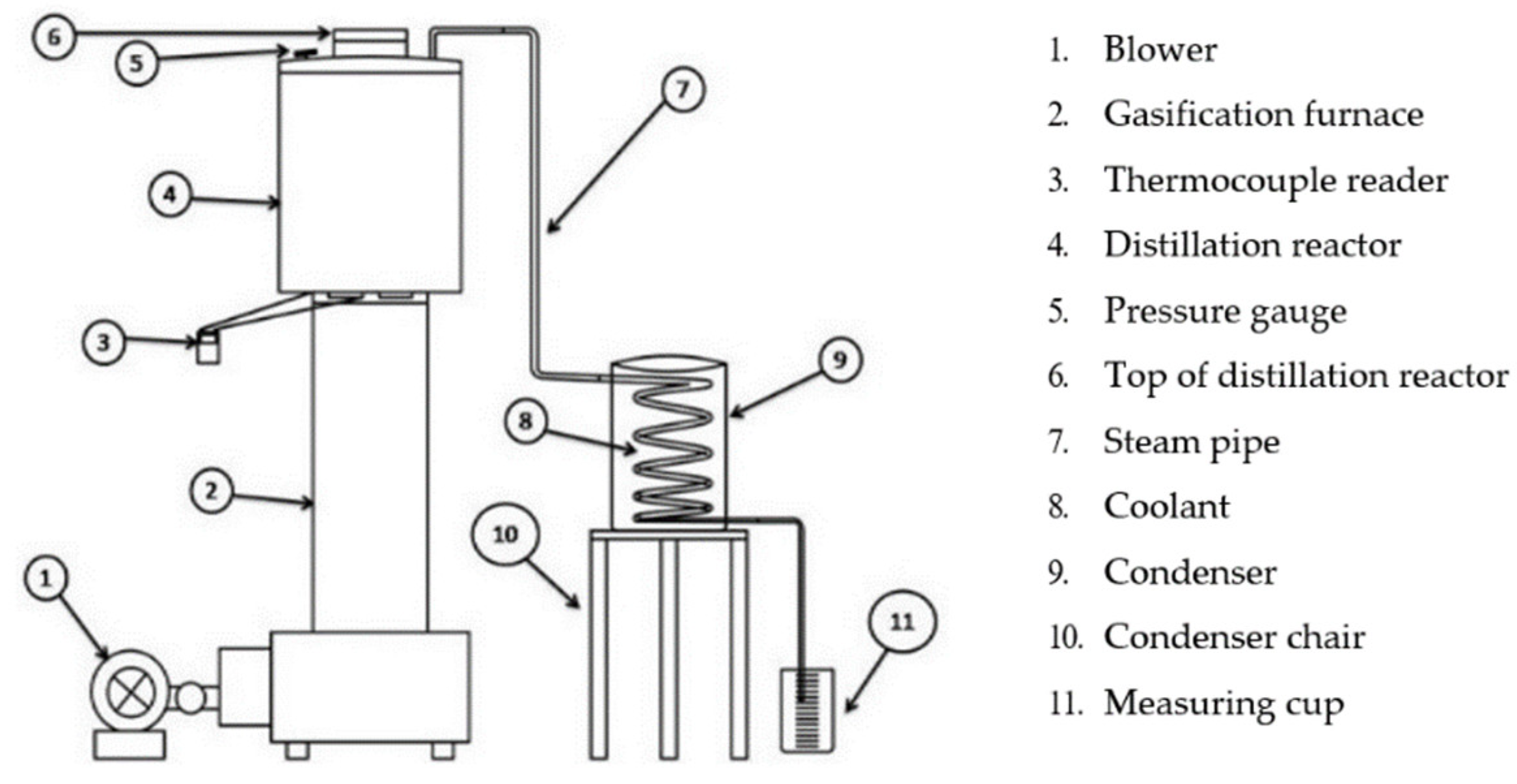
Firstly, we prepared all materials such as rice husk briquettes that had been made and chopped PP plastic waste. After that, we assembled all the research tools as shown in Figure 1.
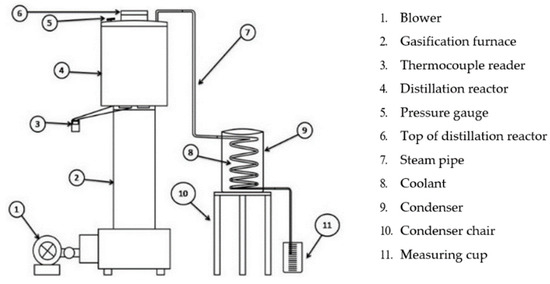
Figure 1.
Installation tools.
Using the TLUD gasification method, by means of turning on the top as a method of burning rice husk briquettes, we loaded 1.7 kg of rice husk briquettes into the gasification stove reactor (2) and turned on the blower as an air supply (1).
If the gasification stove ran out, the procedure was carried out in parallel using 2 stoves that were available to be used as combustion sources until the process was complete.
The test was repeated until the plastic waste pyrolysis process was complete.
After carrying out all the steps to obtain fuel oil, we performed a water boiling test to determine the quality of the oil fuel by comparing it with kerosene.
An analysis of the research results was carried out after the research data were obtained and processed. The subjects of analysis were the graphs obtained from the gasification test of rice husk briquettes and the pyrolysis process of plastic waste.
3. Results and Discussion
3.1. Average Combustion Temperature of Rice Husk Briquette Gasification Using the Stove Six Times
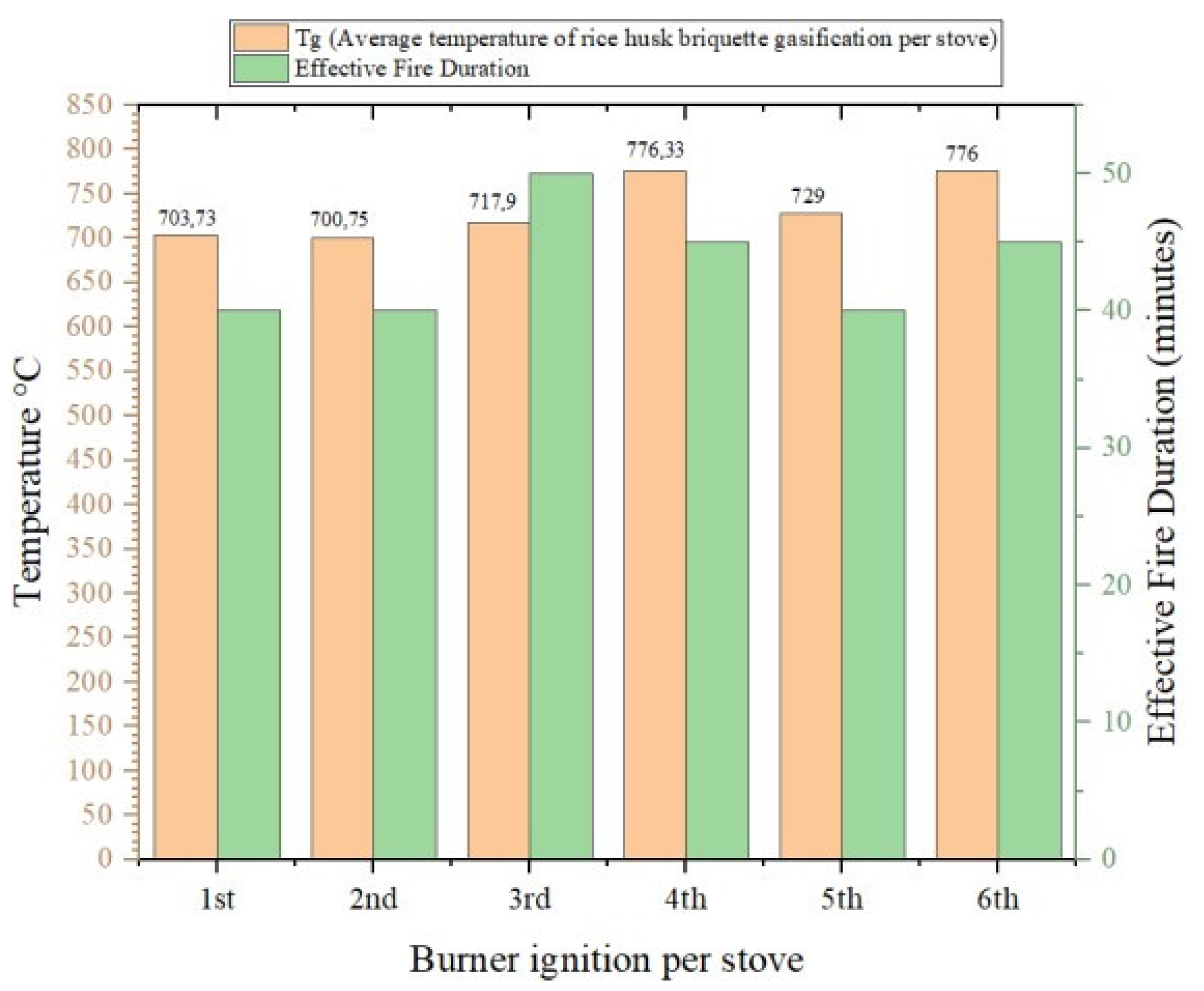
Figure 2 shows the average flame temperature per gasification stove cycle for rice husk briquettes. It can be seen in the graph that the average lit gasification stove was not at a constant temperature. This proves that the flame from the gasification of rice husk briquettes fluctuated. In the first ignition, the average flame was 703.73 °C; then the second ignition was 700.75 °C; in the third ignition, it was 717.9 °C; in the fourth ignition, it was 776.33 °C; in the fifth ignition, it was 729 °C; and in the last ignition, it was 776 °C. Thus, from the six ignitions of the rice husk briquette gasification stove, the average temperature for six ignitions with a duration of 260 min was 733.893 °C. In the first ignition, the effective flame was 40 min; in the second ignition, it was 40 min; in the third ignition, it was 50 min; in the fourth ignition, it was 45 min; in the fifth ignition, it was 40 min and in the sixth ignition, it was 45 min.
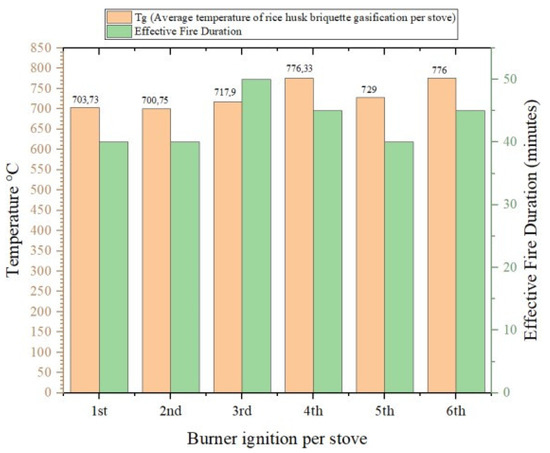
Figure 2.
Diagram of average temperature of rice husk briquette gasification per stove.
3.2. Average Temperature in the Distillation Reactor Section
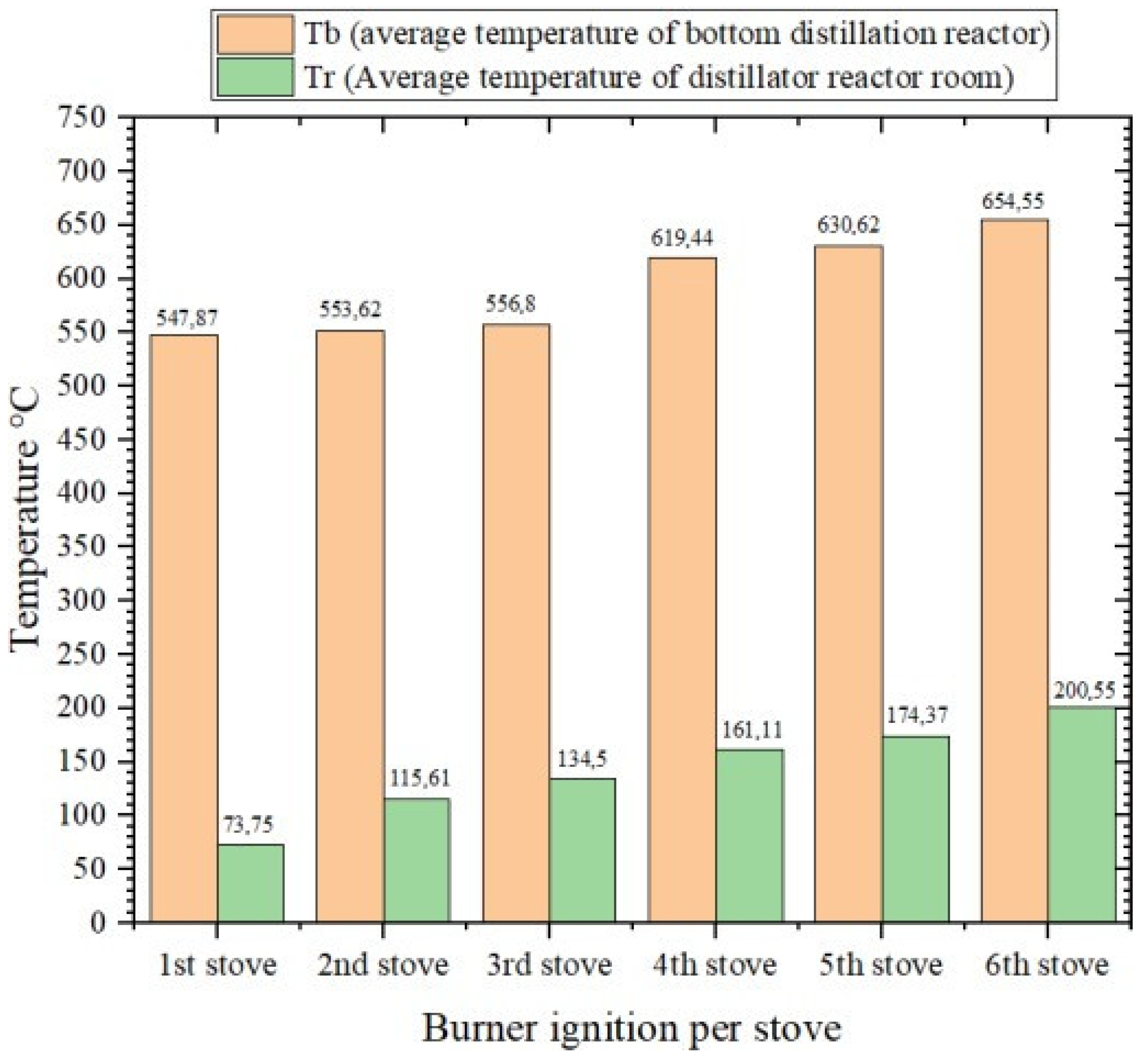
In Figure 3, it can be seen that during the ignition of the six gasification stoves with a duration of 260 min, the average temperature in the distillation reactor section always increased per ignition of the briquette husk gasification stove. At the first ignition, the average temperature at the bottom of the reactor was 547.87 °C, and the average temperature at the reactor chamber was 73.75 °C. In the second ignition, the average temperatures at the bottom of the reactor and the reactor chamber were 552.62 °C and 115.61 °C, respectively. Then the averages of the third ignition were 556.8 °C and 134.5 °C. On the fourth ignition, they were 619.44 °C and 161.11 °C. Then, on the fifth ignition, they were 630.625 °C and 174.37 °C. Finally, on the sixth ignition, they were 654.55 °C and 200.55 °C.
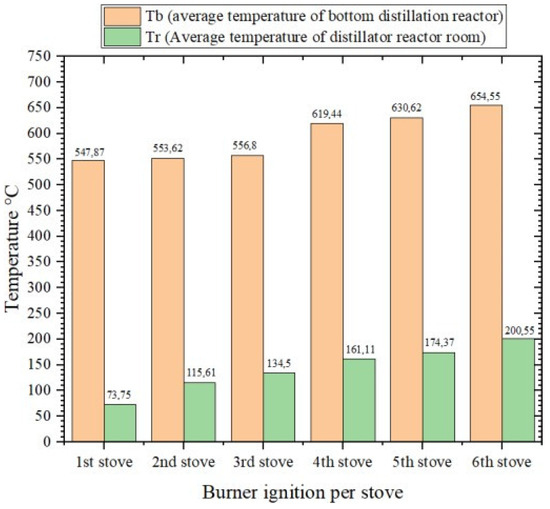
Figure 3.
Diagram Comparison of the average temperature in the distillation reactor.
3.3. Total Mass of Fuel Produced from the Pyrolysis Process
From 3000 g of chopped PP (polypropylene) plastic waste used in this study, during the 260 min pyrolysis process, 1.29 L of fuel oil was obtained as shown by Figure 4. Meanwhile, the tar (residual) produced was 1242 g, with the percentages yielded by this process being 34.4% into fuel oil and 41.4% into tar (residual); 24.2% evaporated (melted) or was able to produce oil with a ratio of 1:3. The oil result can be seen below.

Figure 4.
Oil result.
3.4. Water Boiling Test Result
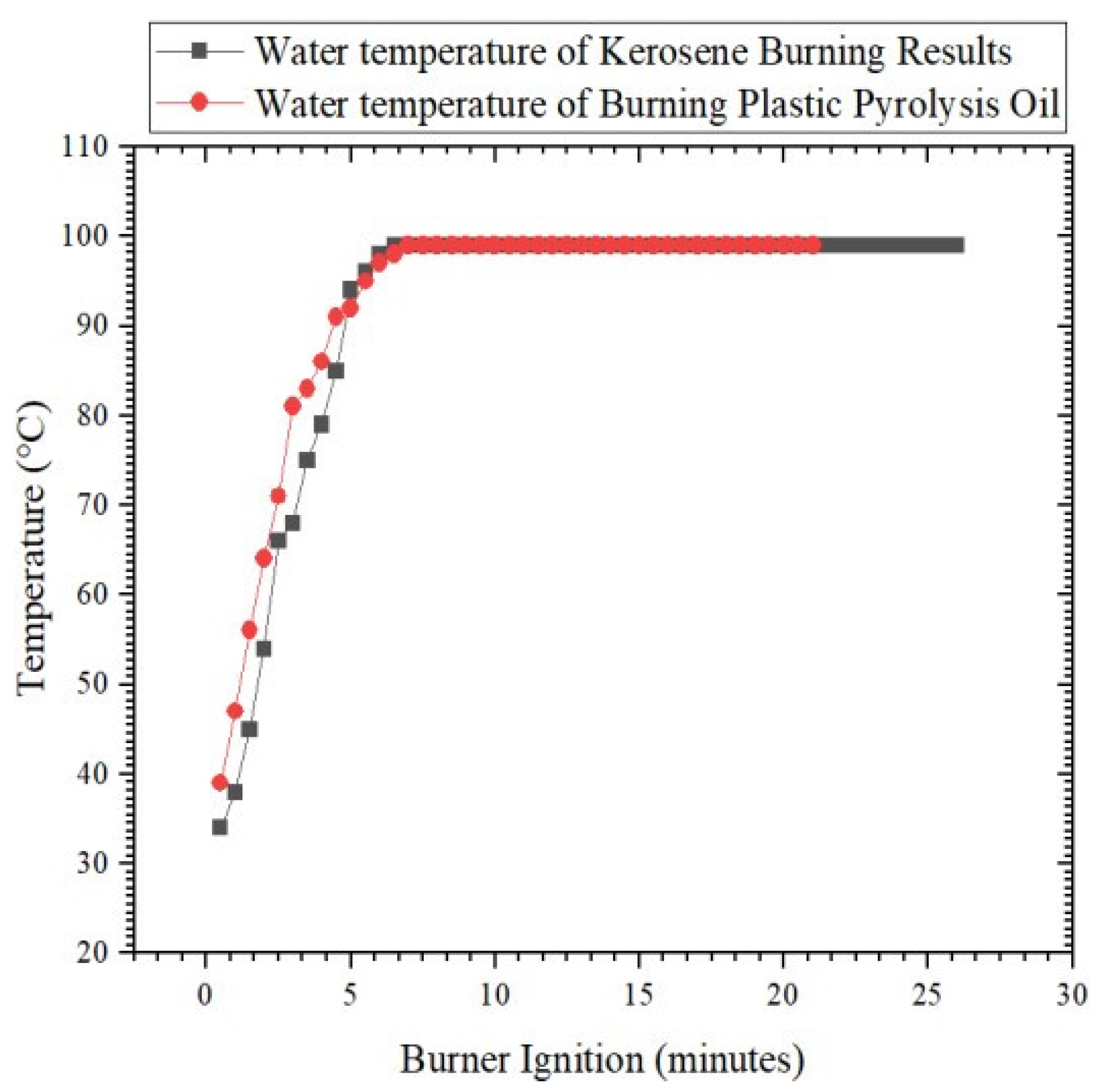
Figure 5 explains that the quality of the fuel oil produced by PP plastic pyrolysis was tested using the water boiling test. The results from boiling water with a mass of 1 kg using 100 mL of kerosene fuel revealed that the highest water temperature reached was 99 °C at 6.5 min, and this temperature was stable for up to 26 min. In testing with plastic pyrolysis fuel oil, the highest water temperature was 99 °C at 7 min, and this temperature was stable for up to 21 min. Then, the temperature began to decrease as the flame that began to fall. Ignition with kerosene fuel lasted for 51 min 32 s, while ignition with fuel oil lasted for 38 min 30 s. During that time, the burning kerosene produced 65 g of water, and 464 g of pyrolysis oil was burned.
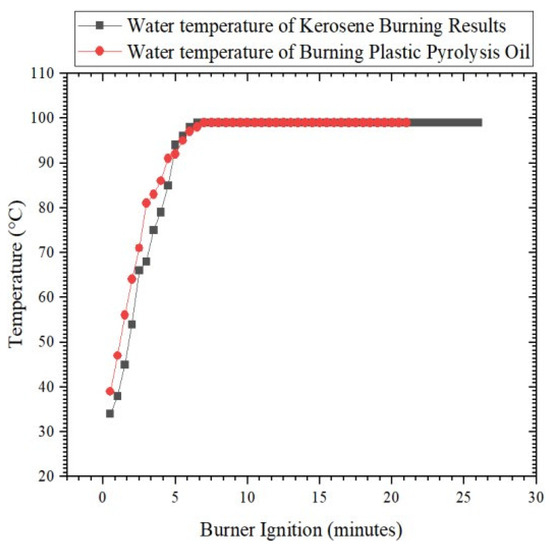
Figure 5.
Graph Comparison of water boiling test between kerosene and pyrolysis oil.
From the calculation of the water boiling test, the sensible heat of the kerosene was 71 Kcal, and that of the pyrolysis oil was 71 Kcal. Additionally, the latent heat produced by the kerosene was 504.9 Kcal, and that of the PP plastic pyrolysis oil was 289.44 Kcal. The heat energy input for the kerosene was 817.35 Kcal, and for the pyrolysis oil, it was 933.6 Kcal. Therefore, the thermal efficiency produced by kerosene was 70.45% and the thermal efficiency produced by the pyrolysis oil was 38.60%.
4. Conclusions
Rice husk briquettes gasification can be used to fuel the pyrolysis process of PP (polypropylene) plastic waste. The combustion was carried out six times in parallel using the gasification furnace. The time used in the pyrolysis process was 260 min with the average effective flame duration of the furnace being 43.33 min, while the average flame temperature recorded across the six times of combustion was 733.89 °C.
In the reactor section, two parameters were measured, namely the temperature of the bottom of the reactor and the temperature of the reactor room. From the six activations of the furnace in parallel, the average temperature at the bottom of the reactor was 593.56 °C, and the average temperature in the reactor chamber was 143.32 °C. This process is categorized as low-temperature pyrolysis
From the 3000 g of chopped PP (Polypropylene) plastic waste used in this study, during the 260-min pyrolysis process, 1.29 L of fuel oil was obtained. Meanwhile, the tar (residual) produced was 1242 g, so that from 3000 g of plastic waste used, the percentage yields of this process were 34.4% into fuel oil, 41.4% into tar (residual), 24.2% evaporated (melts) or was able to produce oil with a ratio of 1:3.
The quality of the fuel oil produced by PP plastic pyrolysis was tested by performing the water boiling test. It was tested with a mass of 1 kg of water and 100 mL of fuel. The result of the testing with kerosene fuel was that the highest water temperature reached was 99 °C at 6.5 min, and this temperature was stable for up to 26 min. In testing with plastic pyrolysis fuel oil, the highest water temperature was 99 °C at 7 min, and this temperature stable for up to 21 min. Then, the temperature began decrease as the flame that began to fall. Ignition with kerosene fuel lasted for 51 min 32 s, while ignition with fuel oil lasted for 38 min 30 s. During that time, the burning kerosene produced 65 g of water, and 464 g of pyrolysis was oil burned.
From the results of the water boiling test between kerosene and pyrolysis oil, it can be seen that the values of sensible heat, latent heat, and heat energy input have values that are not much different. However, kerosene still has better thermal efficiency than the thermal efficiency of pyrolysis oil with a thermal efficiency of 70.45% and 38.60% respectively.
Author Contributions
W. and W.H. worked collaboratively to conduct experiments, analyze data, and draft the manuscript. Both researchers actively participated in the research process and conducted laboratory experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Directorate General of Research, Technology, and Higher Education, Ministry of Education and Culture of the Republic of Indonesia. By the scheme flagship, Regular Fundamental Research (PFR) financial year 2023 with contract number is 006/LL6/PB/AL.04/2023, 170.28/C.1-III/LRI/VI/2023 on 20 June 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated; all data were presented within the manuscript.
Acknowledgments
The author would like to express his deepest gratitude and appreciation to the Directorate General of Research, Technology, and Higher Education, Ministry of Education and Culture of the Republic of Indonesia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baharudin, H.; Windarta, J.; Giovanni, E.H. Konversi Limbah Plastik Menjadi Bahan Bakar. J. Energi Baru Dan Terbarukan 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Landi, T.; Arijanto, A. Perancangan Dan Uji Alat Pengolah Sampah Plastik Jenis Ldpe (Low Density Polyethylene) Menjadi Bahan Bakar Alternatif. J. Tek. Mesin Undip. 2017, 5, 1–8. [Google Scholar]
- Nasrun, N.; Kurniawan, E.; Sari, I. Pengolahan Limbah Kantong Plastik Jenis Kresek Menjadi Bahan Bakar Menggunakan Proses Pirolisis. J. Energi Elektr. 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Sharuddin, S.D.; Abnisa, F.; Daud, W.M.; Aroua, M.K. Pyrolysis of plastic waste for liquid fuel production as prospective energy resource. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 334. [Google Scholar] [CrossRef]
- Nasution, A.Y. Analisis Kalor Pada Alat Pengolah Sampah Plastik Dengan Metode Pyrolisis Dengan Perbandingan Tipe Sampah Plastik. Suara Tek. J. Ilm. 2020, 11, 25. [Google Scholar] [CrossRef]
- Raja, A.; Murali, A. Conversion of Plastic Wastes into Fuels. J. Mater. Sci. Eng. B 2011, 1, 8689. [Google Scholar]
- Pujotomo, I. Potensi Pemanfaatan Biomassa Sekam Padi Untuk Pembangkit Listrik Melalui Teknologi Gasifikasi. Energi Kelistrikan 2018, 9, 126–135. [Google Scholar] [CrossRef]
- Dhanak, D.V.; Patel, V.R. Biomass Gasification: A Modern Approach for Renewable Energy Utilization. GRD J.-Glob. Res. Dev. J. Eng. 2016, 1, 58–65. Available online: www.grdjournals.com (accessed on 5 March 2024).
- Subroto; Wijianto; Sarjit; Himawanto, D.A. Continuous and uncontinuous gasification systems of rice husk using variation modification of burner. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 403. [Google Scholar] [CrossRef]
- Suprianto, F.D.; Gotama, G.J.; Evander, J.; Kasrun, A. Investigation on biomass briquette from Cerbera manghas waste twigs as renewable energy source. ARPN J. Eng. Appl. Sci. 2018, 13, 1080–1084. [Google Scholar]
- Surono, U.B. Berbagai Metode Konversi Sampah Plastik Menjadi Bahan Bakar Minyak. J. Tek. 2013, 3, 31. [Google Scholar]
- Ridhuan, K.; Irawan, D. Buku Energi Terbarukan Pirolisis; CV. Laduny Alifatama: Metro, Indonesia, 2020. [Google Scholar]
- Belonio, A.T. Rice Husk Gas Stove Handbook. Bioenergylists.Org. 2005, p. 155. Available online: http://bioenergylists.org/stovesdoc/Belonio/Belonio_gasifier.pdf (accessed on 5 March 2024).
- Adoe, D.G.; Bunganaen, W.; Krisnawi, I.F.; Soekwanto, F.A. Pirolisis Sampah Plastik PP (Polyprophylene) menjadi Minyak Pirolisis sebagai Bahan Bakar Primer. LONTAR J. Tek. Mesin Undana 2016, 3, 17–26. [Google Scholar]
- Wahyudi, J.; Prayitno, H.T.; Astuti, A.D. Pemanfaatan Limbah Plastik Sebagai Bahan Baku Pembuatan Bahan Bakar Alternatif. J. Litbang 2018, 14, 58–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).