Aptamer-Based Biosensor Design for Simultaneous Detection of Cervical Cancer-Related MicroRNAs †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- At least one valid secondary structure (folding variant) with negative ΔG for aptamers corresponding to 14 circulating miRNAs. Negative ΔG values suggest that these aptamers are likely to form stable secondary structures; the folding process of RNA aptamers is spontaneous and thermodynamically favored.
- At least one folding variant with positive ΔG for 19 circulating miRNAs and no folding secondary structure variants with negative ΔG. This indicates that for these miRNAs, the secondary structures of the aptamers might not be as thermodynamically stable, which could influence their binding kinetics and specificity.
- In the case of three aptamers, the folding process was not possible, and the prediction of the secondary structure failed, namely the corresponding aptamers for miR-138-1-3p (a single aptamer sequence predicted), miR-328-5p (two aptamer sequences predicted), and miR-466 (seven aptamer sequences predicted). This suggests that these particular miRNAs might pose challenges in terms of aptamer design due to their structural complexity or other factors.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Wu, Q.; Hao, Y.; Hu, J.; Gao, Y.; Zhou, S.; Han, L. Global, Regional, and National Burden of Cervical Cancer for 195 Countries and Territories, 2007–2017: Findings from the Global Burden of Disease Study 2017. BMC Women’s Health 2021, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Q.; Khan, Q.A.; Luo, Z. Advances in Optical Aptasensors for Early Detection and Diagnosis of Various Cancer Types. Front. Oncol. 2021, 11, 632165. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dulta, K.; Nagraik, R.; Dua, K.; Singh, S.K.; Chellappan, D.K.; Kumar, D.; Shin, D.-S. Potentialities of Aptasensors in Cancer Diagnosis. Mater. Lett. 2022, 308, 131240. [Google Scholar] [CrossRef]
- Kher, G.; Trehan, S.; Misra, A. Antisense Oligonucleotides and RNA Interference. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Misra, A., Ed.; Elsevier: London, UK, 2011; pp. 325–386. ISBN 978-0-12-384964-9. [Google Scholar]
- Abbas, M.; Mehdi, A.; Khan, F.H.; Verma, S.; Ahmad, A.; Khatoon, F.; Raza, S.T.; Afreen, S.; Glynn, S.A.; Mahdi, F. Role of MiRNAs in Cervical Cancer: A Comprehensive Novel Approach from Pathogenesis to Therapy. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102159. [Google Scholar] [CrossRef] [PubMed]
- Doghish, A.S.; Ali, M.A.; Elyan, S.S.; Elrebehy, M.A.; Mohamed, H.H.; Mansour, R.M.; Elgohary, A.; Ghanem, A.; Faraag, A.H.I.; Abdelmaksoud, N.M.; et al. MiRNAs Role in Cervical Cancer Pathogenesis and Targeted Therapy: Signaling Pathways Interplay. Pathol.—Res. Pract. 2023, 244, 154386. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Lima, L.J.; Morais, A.H.F.; Valentim, R.A.M.; Barreto, E.J.S.S. MiRNAs as Biomarkers for Early Cancer Detection and Their Application in the Development of New Diagnostic Tools. BioMedical Eng. OnLine 2021, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- RNAcentral Consortium. RNAcentral 2021: Secondary Structure Integration, Improved Sequence Search and New Member Databases. Nucleic Acids Res. 2021, 49, D212–D220. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Hoffert, J.D.; Saeed, F.; Knepper, M.A. NHLBI-AbDesigner: An Online Tool for Design of Peptide-Directed Antibodies. Am. J. Physiol. Cell Physiol. 2012, 302, C154–C164. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Zuber, J.; Schroeder, S.J.; Sun, H.; Turner, D.H.; Mathews, D.H. Nearest Neighbor Rules for RNA Helix Folding Thermodynamics: Improved End Effects. Nucleic Acids Res. 2022, 50, 5251–5262. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Aptamer (Predicted) Sequence (N1–Nn) | Aptamer Folding—Secondary Structure (ΔG in kcal/mol) |
|---|---|---|
| miR-10b | CGAUUCUAGGGGAAU (8–22) UCGAUUCUAGGGGAA (7–21) | 3xST: −0.5/−0.2/0.4 3xST: 0.4/0.7/1.0 |
| miR-15b-3p | CAUUAUUUGCUGCUC (6–20) | 2xST: 2.0/2.8 |
| miR-15b-5p | AGCAGCACAUCAUGG (2–16) | 2xST: 0.9/1.9 |
| miR-17-3pPC | CUGCAGUGAAGGCAC (2–16) | 2xST: −1.1/−0.2 |
| miR-17-5pPC | GCUUACAGUGCAGGU (7–21) UGCUUACAGUGCAGG (6–20) GUGCUUACAGUGCAG (5–19) | −1.2 −1.9 4xST: −2.7/−2.5/−2.5/−2.1 |
| miR-21BC | CAGACUGAUGUUGAC (9–23) | −0.4 |
| miR-27b-3p | GUGGCUAAGUUCUGC (7–21) | 3xST: 0.2/1.0/1.1 |
| miR-27b-5p | AGCUGAUUGGUGAAC (8–22) | −0.2 |
| miR-32-3p | AGUGUGUGUGAUAUU (7–21) UAGUGUGUGUGAUAU (6–20) UUAGUGUGUGUGAUA (5–19) AUUUAGUGUGUGUGA (3–17) AAUUUAGUGUGUGUG (2–16) | 1.0 1.3 3xST: 2.80/3.3/3.3 6xST: 3.2/3.5/3.7/4.0/4.1/4.2 3xST: 2.9/3.2/3.7 |
| miR-32-5p | CAUUACUAAGUUGCA (8–22) ACAUUACUAAGUUGC (7–21) CACAUUACUAAGUUG (6–20) GCACAUUACUAAGUU (5–19) UGCACAUUACUAAGU (4–18) UUGCACAUUACUAAG (3–17) | 3xST: 2.8/3.2/3.7 3xST: 2.3/2.3/3.2 2xST: 2.0/2.7 2xST: 2.0/2.9 2xST: 2.1/2.8 2xST: 3.2/4.2 |
| miR-124-3pPC | GGCACGCGGUGAAUG (4–18) | 2xST: 0.1/1.1 |
| miR-124-5pPC | GUGUUCACAGCGGAC (2–16) CGUGUUCACAGCGGA (1–15) | −0.9 2xST: −1.1/−0.5 |
| miR-130a-3p | GCAAUGUUAAAAGGG (5–19) | 4xST: 3.6/4.1/4.5/4.5 |
| miR-130a-5p | UCACAUUGUGCUACU (8–22) UUCACAUUGUGCUAC (7–21) | 0.9 0.9 |
| miR-138-1-3p | CACAACACCAGGGCC (8–22) | No folding is possible |
| miR-138-2-3p | CACGACACCAGGGUU (8–22) UCACGACACCAGGGU (7–21) UUCACGACACCAGGG (6–20) | 2xST: 0.6/0.7 0.7 4xST: 2.9/3.4/3.7/3.9 |
| miR-138-5p | GUGUUGUGAAUCAGG (6–20) GGUGUUGUGAAUCAG (5–19) AGCUGGUGUUGUGAA (1–15) | 2xST: 0.9/1.5 −0.1 2xST: 0.3/0.9 |
| miR-143-3pPC | GAUGAAGCACUGUAG (4–18) GAGAUGAAGCACUGU (2–16) UGAGAUGAAGCACUG (1–15) | 5xST: 2.7/2.9/3.2/3.3/3.7 2xST: 1.8/2.5 3xST: 1.8/2.2/2.5 |
| miR-143-5pPC | GGUGCAGUGCUGCAU (1–15) | −4.5 |
| miR-146a | ACUGAAUUCCAUGGG (7–21) | 5xST: 1.3/1.5/1.8/1.8/2.5 |
| miR-192-3p | UUCCAUAGGUCACAG (8–22) GCCAAUUCCAUAGGU (3–17) UGCCAAUUCCAUAGG (2–16) | 1.7 −0.4 2xST: 1.4/2.3 |
| miR-192-5p | UAUGAAUUGACAGCC (7–21) CUAUGAAUUGACAGC (6–20) CCUAUGAAUUGACAG (5–19) GACCUAUGAAUUGAC (3–17) | 1.6 1.6 1.6 2xST: 3.1/4.0 |
| miR-214 | GGCACAGACAGGCAG (7–21) GCAGGCACAGACAGG (4–18) | −0.9 No folding is possible |
| miR-218-1-3p | CGUCAAGCACCAUGG (8–22) | 7xST: 1.4/1.4/1.7/2.1/2.2/2.3/2.3 |
| miR-218-2-3p | CUGUCAAGCACCGCG (8–22) | 3xST: 0.4/1.2/1.3 |
| miR-218-5p | GUGCUUGAUCUAACC (3–17) | 2.1 |
| miR-328-3p | GGCCCUCUCUGCCCU (3–17) UGGCCCUCUCUGCCC (2–16) CUGGCCCUCUCUGCC (1–15) | −2.4 −2.4 −1.6 |
| miR-328-5p | GGGGGGCAGGAGGGG (2–16) GGGGGGGCAGGAGGG (1–15) | No folding is possible No folding is possible |
| miR-409-3p | GAAUGUUGCUCGGUG (1–15) | 2xST: 1.3/2.1 |
| miR-409-5p | GGUUACCCGAGCAAC (2–16) | 2xST: −0.40/0.2 |
| miR-429 | GUCUGGUAAAACCGU (8–22) UGUCUGGUAAAACCG (7–21) | 3xST: −1.3/−1.2/−1.0 3xST: −1.3/−1.0/−0.6 |
| miR-432-3p | CUGGAUGGCUCCUCC (1–15) | −1.6 |
| miR-432-5p | GAGUAGGUCAUUGGG (6–20) GGAGUAGGUCAUUGG (5–19) | 1.3 2xST: 1.3/1.9 |
| miR-454-3p | AUAUUGCUUAUAGGG (8–22) | 4xST: 1.4/1.8/1.9/2.4 |
| miR-454-5p | CAAUAUUGUCUCUGC (8–22) | 3xST: 2.6/3.1/3.5 |
| miR-466 | ACACGCAACACACAU (9–23) UACACGCAACACACA (8–22) AUACACGCAACACAC (7–21) CAUACACGCAACACA (6–20) ACAUACACGCAACAC (5–19) CACAUACACGCAACA (4–18) ACACAUACACGCAAC (3–17) | No folding is possible No folding is possible No folding is possible No folding is possible No folding is possible No folding is possible No folding is possible |
| Folding of Predicted Sequence | Thermodynamics of Folding | ||
|---|---|---|---|
Sequence: CAGACUGAUGUUGAC | Structure ΔG = −0.4 kcal/mol | ||
| Structural element | δG | Information | |
| External loop | −0.5 | 5 ss bases and 1 closing helix | |
| Stack | −1.3 | External closing pair is G3–U12 | |
| Stack | −2.2 | External closing pair is A4–U11 | |
| Helix | −3.5 | 3 base pairs | |
| Hairpin loop | 3.6 | Closing pair is C5–G10 | |
| Folding of Predicted Sequence | Thermodynamics of Folding | ||
|---|---|---|---|
Sequence 1: GUGUUCACAGCGGAC | Structure ΔG = −0.9 kcal/mol | ||
| Structural element | δG | Information | |
| External loop | 0.0 | 2 ss bases and 1 closing helix | |
| Stack | −2.2 | External closing pair is G3–C15 | |
| Stack | −1.3 | External closing pair is U4–A14 | |
| Stack | −1.5 | External closing pair is U5–G13 | |
| Helix | −5.0 | 4 base pairs | |
| Hairpin loop | 4.1 | Closing pair is C6–G12 | |
Sequence 2: CGUGUUCACAGCGGA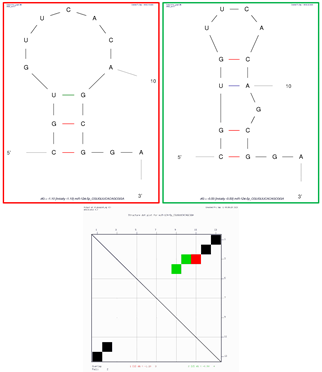 | Structure 1 (red on dot plot folding comparison) ΔG = −1.1 kcal/mol | ||
| Structural element | δG | Information | |
| External loop | −1.3 | 2 ss bases and 1 closing helix | |
| Stack | −2.4 | External closing pair is C1–G13 | |
| Stack | −2.5 | External closing pair is G2–C12 | |
| Helix | −4.9 | 3 base pairs | |
| Hairpin loop | 5.1 | Closing pair is U3–G11 | |
| Structure 2 (green on dot plot folding comparison) ΔG = −0.5 | |||
| Structural element | δG | Information | |
| External loop | −1.3 | 2 ss bases and 1 closing helix | |
| Stack | −2.4 | External closing pair is C1–G13 | |
| Helix | −2.4 | 2 base pairs | |
| Bulge loop | 1.6 | External closing pair is G2–C12 | |
| Stack | −2.1 | External closing pair is U3–A10 | |
| Helix | −2.1 | 2 base pairs | |
| Hairpin loop | 3.7 | Closing pair is G4–C9 | |
| Folding of Predicted Sequence | Thermodynamics of Folding | ||
|---|---|---|---|
Sequence: ACUGAAUUCCAUGGG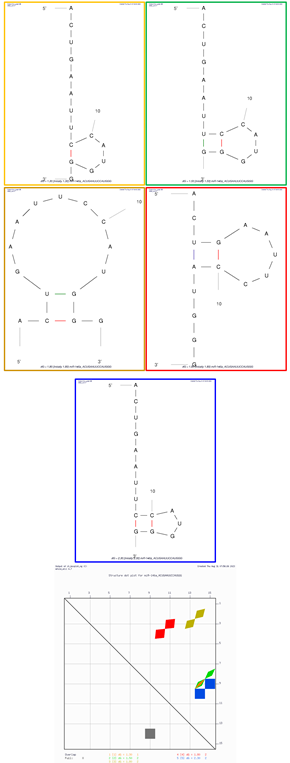 | Structure 1 (orange on dot plot folding comparison) ΔG = 1.3 kcal/mol | ||
| Structural element | δG | Information | |
| External loop | −1.4 | 9 ss bases and 1 closing helix | |
| Hairpin loop | 2.7 | Closing pair is C9–G14 | |
| Structure 2 (green on dot plot folding comparison) ΔG = 1.5 kcal/mol | |||
| Structural element | δG | Information | |
| External loop | 0.3 | 7 ss bases and 1 closing helix | |
| Stack | −1.5 | External closing pair is U8–G15 | |
| Helix | −1.5 | 2 base pairs | |
| Hairpin loop | 2.7 | Closing pair is C9–G14 | |
| Structure 3 (dark gold on dot plot folding comparison) ΔG = 1.8 kcal/mol | |||
| Structural element | δG | Information | |
| External loop | −1.8 | 2 ss bases and 1 closing helix | |
| Stack | −2.1 | External closing pair is C2–G14 | |
| Helix | −2.1 | 2 base pairs | |
| Hairpin loop | 5.7 | Closing pair is U3–G13 | |
| Structure 4 (red on dot plot folding comparison) ΔG = 1.8 kcal/mol | |||
| Structural element | δG | Information | |
| External loop | −0.2 | 6 ss bases and 1 closing helix | |
| Stack | −2.1 | External closing pair is U3–A11 | |
| Helix | −2.1 | 2 base pairs | |
| Hairpin loop | 4.1 | Closing pair is G4–C10 | |
| Structure 5 (blue on dot plot folding comparison) ΔG = 2.5 kcal/mol | |||
| Structural element | δG | Information | |
| External loop | −0.1 | 8 ss bases and 1 closing helix | |
| Stack | −3.3 | External closing pair is C9–G15 | |
| Helix | −3.3 | 2 base pairs | |
| Hairpin loop | 5.7 | Closing pair is C10–G14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaian, R. Aptamer-Based Biosensor Design for Simultaneous Detection of Cervical Cancer-Related MicroRNAs. Eng. Proc. 2023, 58, 88. https://doi.org/10.3390/ecsa-10-16203
Tamaian R. Aptamer-Based Biosensor Design for Simultaneous Detection of Cervical Cancer-Related MicroRNAs. Engineering Proceedings. 2023; 58(1):88. https://doi.org/10.3390/ecsa-10-16203
Chicago/Turabian StyleTamaian, Radu. 2023. "Aptamer-Based Biosensor Design for Simultaneous Detection of Cervical Cancer-Related MicroRNAs" Engineering Proceedings 58, no. 1: 88. https://doi.org/10.3390/ecsa-10-16203
APA StyleTamaian, R. (2023). Aptamer-Based Biosensor Design for Simultaneous Detection of Cervical Cancer-Related MicroRNAs. Engineering Proceedings, 58(1), 88. https://doi.org/10.3390/ecsa-10-16203






