Algal Organic Matter Fluorescence Analysis of Chlorella sp. for Biomass Estimation †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Preparation
2.2. Spectral Characterization
2.2.1. Measuring UV-Vis Using Absorbance Spectroscopy
2.2.2. Measuring Fluorescence Using Excitation–Emission Spectroscopy
2.3. The Application of the EEM to Fluorescence Lidar Measurements
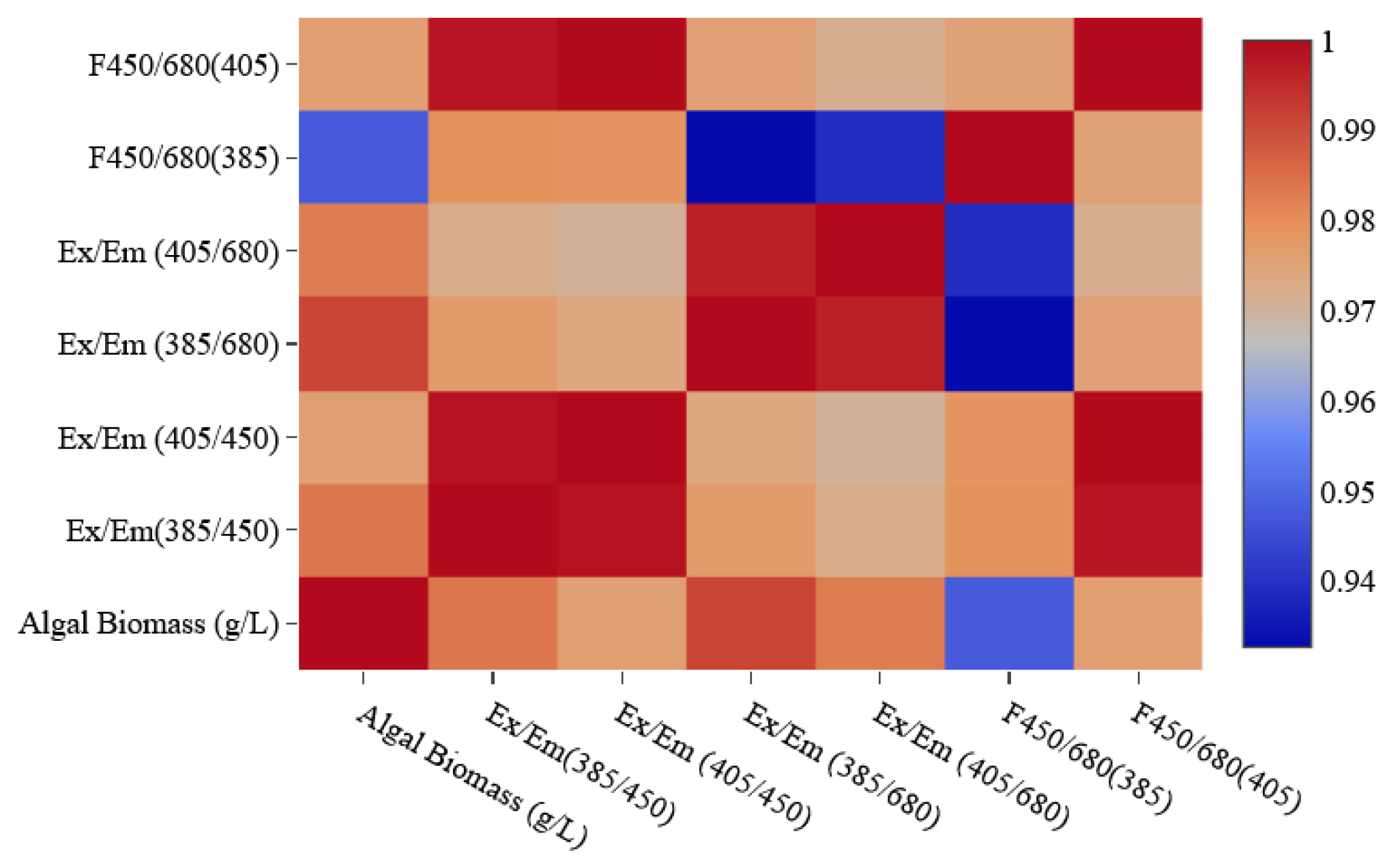
2.4. Comparisons of the Fluorescence EEM
3. Results and Discussion
3.1. The Spectral Characterization of Chlorella sp.
3.2. The Analysis of the Fluorescence EEM regarding the Development of the Lidar System
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebrahimzadeh, G.; Alimohammdi, M.; Kahkah, M.; Mahvi, A.H. Relationship between algae diversity and water quality-a case study: Chah Niemeh reservoir Southeast of Iran. J. Environ. Health Sci. Eng. 2021, 19, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, L.; Zhao, X.; Chen, J.; Li, Z.; Liu, Y.; Ou, L.; Xie, Z.; Wang, M.; Yin, X.; et al. Evaluation of environmental factors and microbial community structure in an important drinking-water reservoir across seasons. Front. Microbiol. 2023, 14, 1091818. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.A.; Garneau, M.E.; Seubert, E.; Howard, M.D.A.; Darjany, L.; Schnetzer, A.; Cetinic, I.; Filteau, G.; Lauri, P.; Jones, B.; et al. Harmful algae and their potential impacts on desalination operations off southern California. Water Res. 2010, 44, 385–416. [Google Scholar] [CrossRef] [PubMed]
- Villacorte, L.O.; Ekowati, Y.; Neu, T.R.; Kleijn, J.M.; Winters, H.; Amy, G.; Schippers, J.C.; Kennedy, M.D. Characterisation of algal organic matter by bloom-forming marine and freshwater algae. Water Res. 2015, 73, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; An, S.; He, H.; Wen, S.; Xing, P.; Duan, H. Production and transformation of organic matter driven by algal blooms in a shallow lake: Role of sediments. Water Res. 2022, 219, 118560. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Yu, H.; Tan, X.; Zhang, Y.; Zhou, X.; Yang, L.; Li, D. Extraction procedure optimization and the characteristics of dissolved extracellular organic matter (bEOM) from chlorella pyrenoidosa. Coll. Surf. B Biointerfaces 2015, 125, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhang, Z.; Wang, Z.; Zhang, P.; Xiong, C.; Kuang, Y.; Peng, X.; Yu, M.; Qian, Y. Compositional variations in algal organic matter during distinct growth phases in karst water. Front. Environ. Sci. 2023, 11, 1112522. [Google Scholar]
- Saito, Y.; Hosokawa, T.; Shiraishi, K. Collection of excitation-emission-matrix fluorescence of aerosol-candidate-substances and its application to fluorescence lidar monitoring. Appl. Opt. 2022, 61, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Cadondon, J.G.; Vallar, E.A.; Beltran, A.B.; Orbecido, A.H.; Galvez, M.C.D. Variation in Dissolved Organic Matter Using Absorbance and Fluorescence Measurements during Dry Season in St. Rosa and Cabuyao Rivers, Philippines. Water 2022, 14, 1444. [Google Scholar] [CrossRef]
- Lal, P.P.; Juste-Poinapen, M.S.N.; Poinapen, J. Assessing the water quality of Suva foreshore for the establishment of estuary and marine recreational water guidelines in the Fiji Islands. Water Sci. Technol. 2021, 84, 3040–3054. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, P.; Ray, R.; Paul, M.; Gupta, V.K.; Acharya, A.; Bakshi, S.; Jana, T.K.; Mukhopadhyay, S.K. Assessing the Dynamics of Dissolved Organic Matter (DOM) in the Costal Environments Dominated by Mangroves, Indian Sundarbans. Front. Earth Sci. 2020, 8, 218. [Google Scholar] [CrossRef]
- Cadondon, J.; Vallar, E.; Belo, L.; Orbecido, A.; Galvez, M.C. UV-Vis Absorbance and Fluorescence Characterization of Pasig River Surface Water Samples Towards the Development of an LED Fluorescence Lidar System. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 968–980. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Ong, P.M.B.; Vallar, E.A.; Shiina, T.; Galvez, M.C.D. Chlorophyll-a pigment measurement of spirulina in algal growth monitoring using portable pulsed LED fluorescence lidar system. Sensors 2022, 22, 2940. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Vallar, E.A.; Shiina, T.; Galvez, M.C.D. Real-time Chlorophyll-a Pigment Monitoring of Chlamydomonas reinhardtii in a Controlled Environment Using Pulsed LED Fluorescence LiDAR System. Photonics 2023, 10, 144. [Google Scholar] [CrossRef]
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [PubMed]
- Lee, S.; Park, J. Identification of Dissolved Organic Matter Origin Using Molecular Level Analysis Methods. Water 2022, 14, 1317. [Google Scholar] [CrossRef]
- Harjung, A.; Schweichhart, J.; Rash, G.; Griebler, C. Large-scale study on groundwater dissolved organic matter reveals a strong heterogeneity and a complex microbial footprint. Sci. Total Environ. 2023, 854, 158542. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadondon, J.; Lesidan, J.R.; Bulan, J.; Vallar, E.; Shiina, T.; Galvez, M.C. Algal Organic Matter Fluorescence Analysis of Chlorella sp. for Biomass Estimation. Eng. Proc. 2023, 58, 80. https://doi.org/10.3390/ecsa-10-16220
Cadondon J, Lesidan JR, Bulan J, Vallar E, Shiina T, Galvez MC. Algal Organic Matter Fluorescence Analysis of Chlorella sp. for Biomass Estimation. Engineering Proceedings. 2023; 58(1):80. https://doi.org/10.3390/ecsa-10-16220
Chicago/Turabian StyleCadondon, Jumar, James Roy Lesidan, Jejomar Bulan, Edgar Vallar, Tatsuo Shiina, and Maria Cecilia Galvez. 2023. "Algal Organic Matter Fluorescence Analysis of Chlorella sp. for Biomass Estimation" Engineering Proceedings 58, no. 1: 80. https://doi.org/10.3390/ecsa-10-16220
APA StyleCadondon, J., Lesidan, J. R., Bulan, J., Vallar, E., Shiina, T., & Galvez, M. C. (2023). Algal Organic Matter Fluorescence Analysis of Chlorella sp. for Biomass Estimation. Engineering Proceedings, 58(1), 80. https://doi.org/10.3390/ecsa-10-16220







