Removal of Humic Substances from Water with Granular Activated Carbons †
Abstract
:1. Introduction
- An increase in the intensity of the color of the water;
- An increase the acidity of the water;
- An effect on the smell and taste of water;
- An effect on the formation of metal complexes (e.g., with Fe, Mn, Al, and Cu);
- An effect on the adsorption of organic compounds (e.g., pesticides, PCBs, and phthalates);
1.1. Removal of Humic Substances
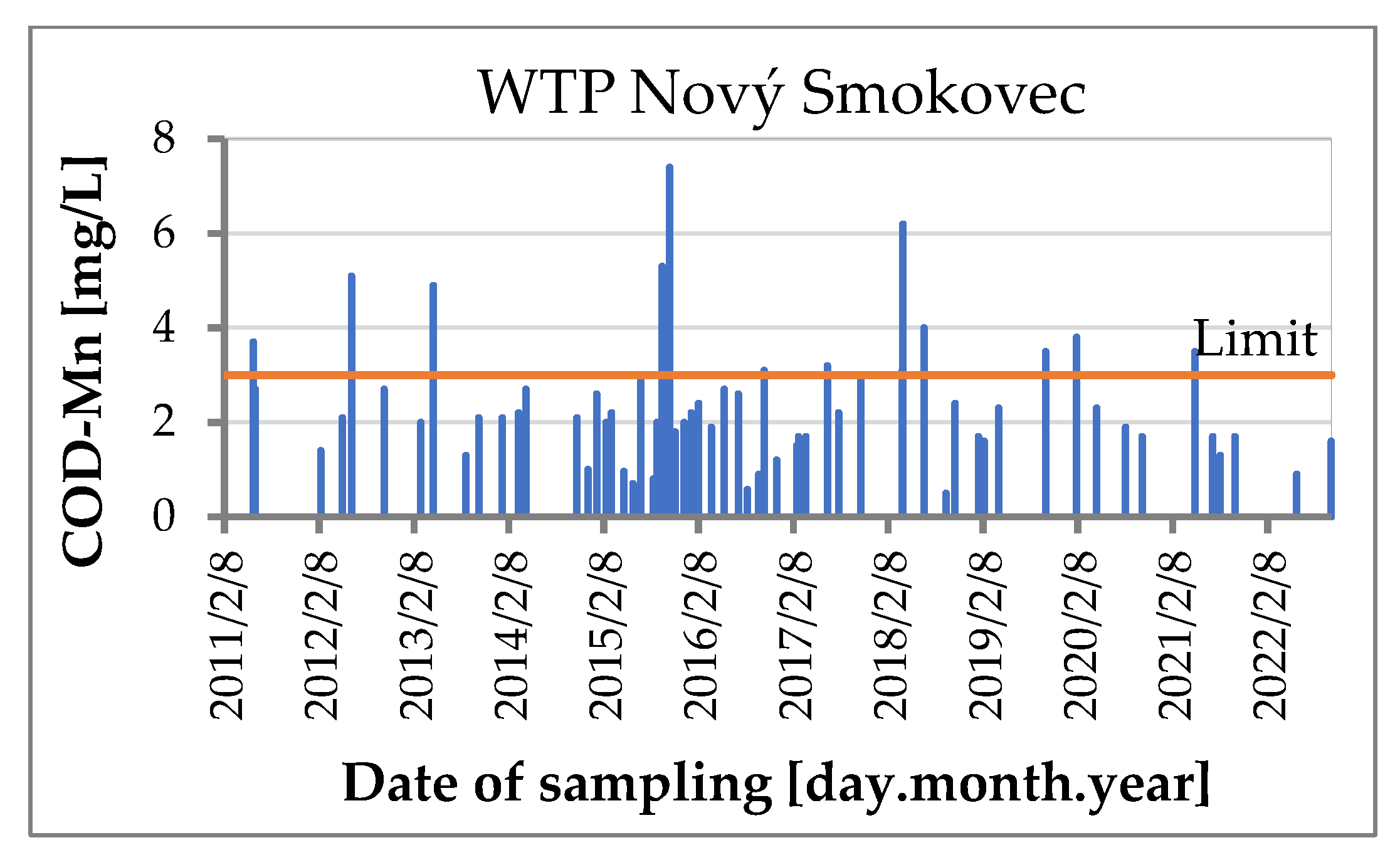
1.2. WTP Nový Smokovec
2. Materials and Methods
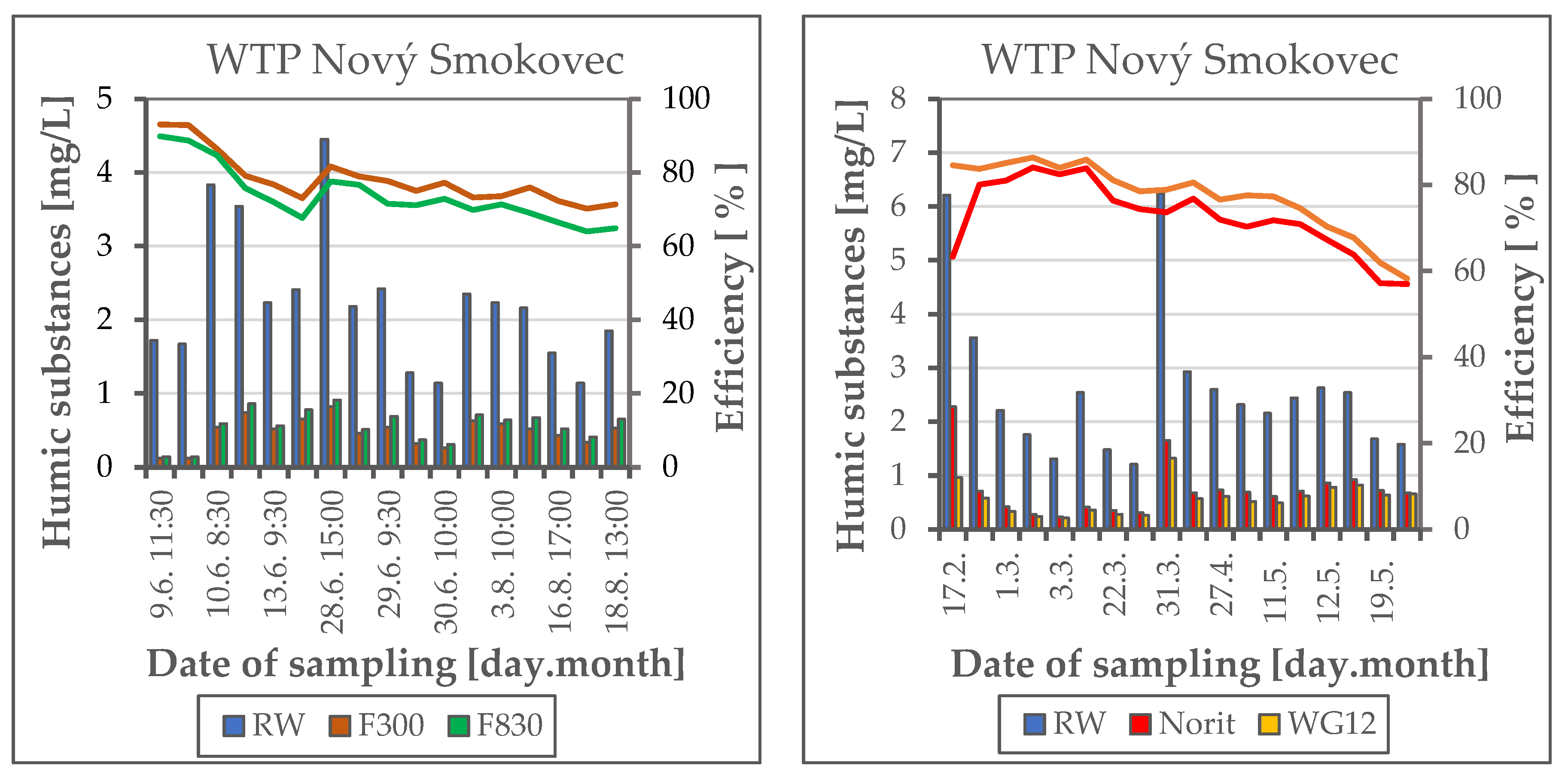
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnitzer, M.; Khan, S.U. Humic Substances in the Environment; M. Dekker: New York, NY, USA, 1972. [Google Scholar]
- Aiken, G.R.; McKnight, D.M.; Wershaw, R.L.; MacCarthy, P. (Eds.) Humic Substances in Soil, Sediment and Water: Geochemistry, Isolation and Characterization; Wiley-Interscience: New York, NY, USA, 1985; 692p. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: New York, NY, USA, 1982; 443p. [Google Scholar]
- MacCarthy, P. The principles of Humic Substances. Soil Sci. 2001, 166, 738–751. [Google Scholar] [CrossRef]
- Suffet, I.H.; MacCarthy, P. (Eds.) Aquatic Humic Substances: Influence on Fate and Treatment of Pollutants; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Pitter, P. Hydrochemie, 5th ed.; Vydavatelství VŠCHT Praha: Praha, Czech Republic, 2015; 792p. (In Czech) [Google Scholar]
- Singer, P.C. Trace Metals and Metal-Organic Interactions in Natural Water; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1973. [Google Scholar]
- Nriagu, J.O.; Coker, R.D. Trace Metals in Humic and Fulvic Acids from Lake Ontario Sediments. Environ. Sci. Technol. 1990, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Snoeying, V.L.; Jenkins, D. Water Chemistry; Wiley: New York, NY, USA, 1991; 480p. [Google Scholar]
- Sillanpää, M. Natural Organic Matter in Water, Characterization and Treatment Methods, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; 382p. [Google Scholar]
- Grünwald, A.; Janda, V.; Fišar, P.; Bížová, J.; Štastný, B. Evaluation of the potential of THM formation in humic waters. In Proceedings of the Conference Voda Zlín 2002, Zlín, Czech Republic, 26–27 March 2002; pp. 67–71. (In Czech). [Google Scholar]
- Clark, R.M.; Thurnau, R.C.; Sivaganesan, M.; Ringhand, P. Prediction the formation of Chlorinated and Brominated By-Products. J. Environ. Eng. 2001, 6, 493–501. [Google Scholar] [CrossRef]
- Decree of the Ministry of Health of the Slovak Republic no. 91/2023 Coll. for Indicators and Limit Values of Drinking Water Quality and Hot Water Quality, Drinking Water Monitoring Procedure, Drinking Water Supply Risk Management, and Risk Management of Domestic Distribution Systems; The Office of the Government of the Slovak Republic: Bratislava, Slovakia, 2023. (In Slovak)
- STN 75 7111; Water Quality, Drinking Water. Slovak Institute for Normalization, Metrology and Testing: Bratislava, Slovakia, 1998. (In Slovak)
- Decree of the Ministry of the Environment of the Slovak Republic no. 636/2004 Coll. Annex 1 about the Requirements for the Quality of Raw Water and Monitoring Water Quality in Water Supply Systems; The Office of the Government of the Slovak Republic: Bratislava, Slovakia, 2023.
- Regulation of the Government of the Slovak Republic No. 269/2010 Coll. Annex 1 and 2, Laying Down the Requirements to Achieve a Good Condition of Water; The Office of the Government of the Slovak Republic: Bratislava, Slovakia, 2022.
- Basumallick, S.; Santra, S. Monitoring of ppm level humic acid in surface water using ZnO–chitosan nanocomposite as fluorescence probe. Appl. Water Sci. 2017, 7, 1025–1031. [Google Scholar] [CrossRef]
- Crittenden, J.; Trussell, R.R.; Hand, D.; Howe, K.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; 1907p. [Google Scholar]
- Binnie, C.; Kimber, M. Basic Water Treatment, 5th ed.; ICE Publishing: London, UK, 2013; 316p. [Google Scholar]
- Yue, Y.; An, G.; Liu, L.; Lin, L.; Jiao, R.; Wang, D. Pre-aggregation of Al13 in optimizing coagulation for removal of humic acid. Chemosphere 2021, 277, 130268. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Alomar, T.; Qiblawey, H.; Almomani, F.; Al-Raoush, R.I.; Han, D.S.; Ahmad, N.M. Recent advances on humic acid removal from wastewater using adsorption process. J. Water Process Eng. 2023, 53, 103679. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.K. Conventional and non-conventional adsorbents for removal of pollutants from water—A review. Indian J. Chem. Technol. 2006, 13, 203–217. [Google Scholar]
- Pertile, E.; Dvorský, T.; Václavík, V.; Heviánková, S. Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution. Life 2021, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Pertile, E.; Václavík, V.; Dvorský, T.; Heviánková, S. The Removal of Residual Concentration of Hazardous Metals in Wastewater from a Neutralization Station Using Biosorbent—A Case Study Company Gutra, Czech Republic. Int. J. Environ. Res. Public Health 2020, 17, 7225. [Google Scholar] [CrossRef] [PubMed]
- Filtrasorb 300: Granular Activated Carbon, CalgonCarbon [online]. 2023. Available online: https://www.calgoncarbon.com/app/uploads/DS-FILTRA30019-EIN-E1.pdf (accessed on 14 August 2023).
- Filtrasorb TL830: Granular Activated Carbon, Chemwiron Carbon [online]. 2023. Available online: https://www.treitelonline.com/wp-content/uploads/2021/03/PDS_FILTRASORB-TL830.pdf (accessed on 2 July 2023).
- Norit GAC 1240: Cabot Corporation, Norit Granulated Activated Carbon. Available online: https://www.ulprospector.com/en/na/Food/Detail/17187/420404/Norit-GAC-1240 (accessed on 15 September 2018).
- Envi-Pur. Granular Activated Carbon [Online]. 2023. Available online: Doc_108_granulovane_aktivni_uhli_wg12_cz.pdf (accessed on 14 August 2023). (In Czech).
- STN 75 7567; Water Quality, Determination of Humic Substances, Photometric Method. Slovak Institute of Technical Normalization: Bratislava, Slovakia, 2007. (In Slovak)
- Standard Methods for the Examination of Water and Wastewater. 5510 Aquatic Humic Substances, 19th ed.; American Public Health Association: Washington, DC, USA, 1995.
- STN EN ISO 8467:2001; Water Quality, Determination of Chemical Oxygen Consumption Permanganate. Slovak Institute for Normalization, Metrology and Testing: Bratislava, Slovakia, 2020.
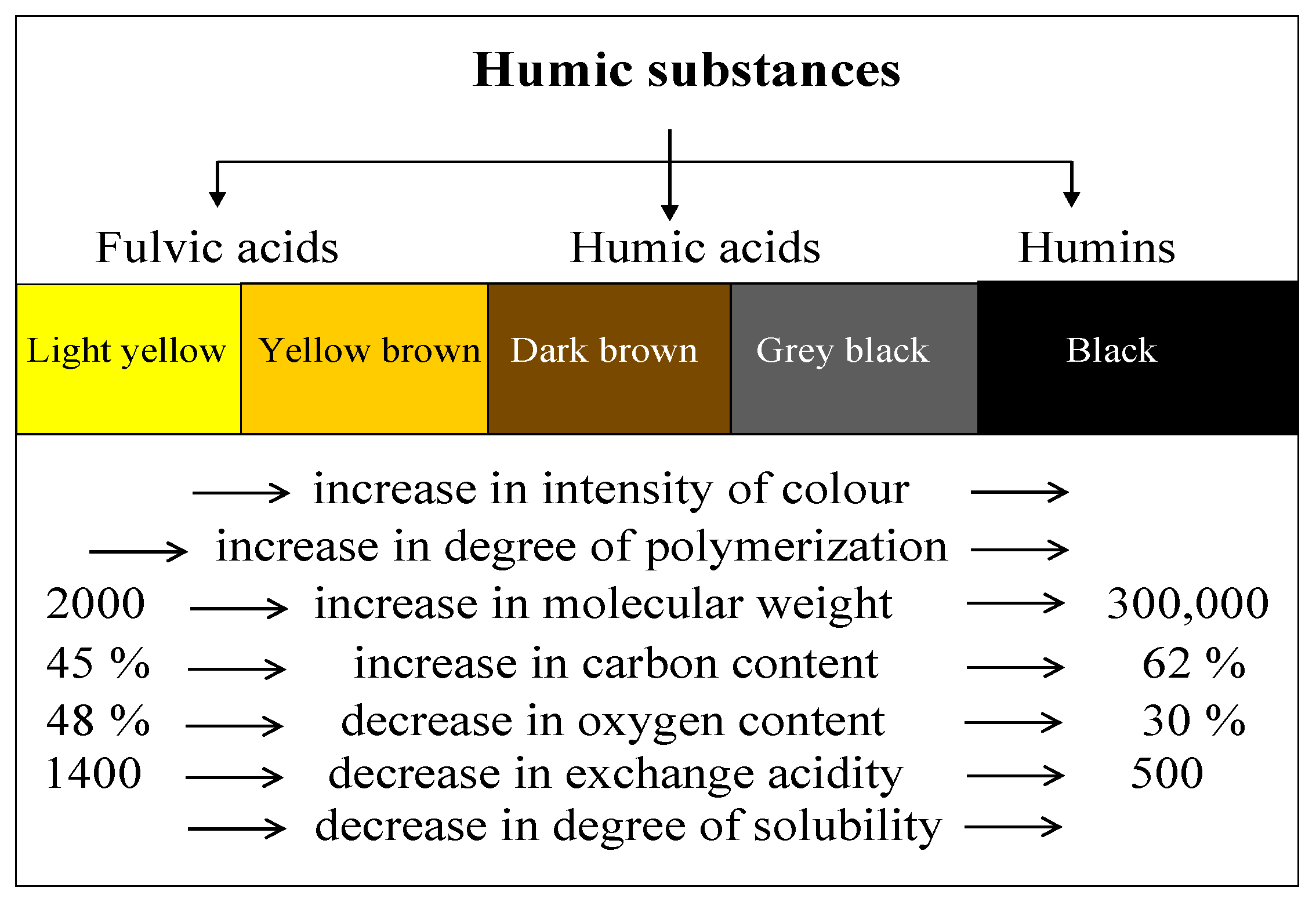


| Humic Substances | Elementary Composition (%) | |||
|---|---|---|---|---|
| C | O | H | N | |
| humic acids | 52–62 | 30–39 | 2.5–5.8 | 2.6–5.1 |
| fulvic acids | 43–52 | 42–51 | 3.3–6.0 | 1.0–6.0 |
| Parameter | Unit | Category A1 | Category A2 | Category A3 | |||
|---|---|---|---|---|---|---|---|
| OH | MH | OH | MH | OH | MH | ||
| CODMn | mg·L−1 | 2 | 3 | 5 | 7 | 8 | 10 |
| TOC | mg·L−1 | <5.0 | 5.0 | 5.0 | 7.0 | 8.0 | 10.0 |
| Turbidity | FNU | 2 | 5 | - | 30 | - | 50 |
| Color | mg·L−1 Pt | 10 | 20 | 50 | 100 | ||
| Absorbance (254 nm) | - | 0.08 | - | 0.15 | - | 0.30 | |
| Parameter | Norit 1240 | WG12 | F300 | F830 |
|---|---|---|---|---|
| Iodine number (mg·g−1) | min. 1020 | min. 1000 | min. 1000 | min. 900 |
| Methylene blue (mg·g−1) | min. 230 | min. 30 | min. 245 | min. 260 |
| Specific surface (BET) (m2·g−1) | 1150 | min 1000 | 1050 | 1100 |
| Particle size (mm) | 0.85–2.0 | 1.0–1.5 | 0.85–2.0 | 0.6–2.36 |
| Median diameter of particles (mm) | 1.4 | 1.2 | 1.4 | 1.6 |
| Operational density (g·gcm−3) | 0.480 | 0.450 ± 30 | 0.450 | 0.460 |
| Abrasion (-) | 75 | 85 | 96 | 95 |
| Hardness (-) | 97 | 95 | 75 | 78 |
| Coefficient of uniformity | 1.6 | 1.3 | 1.4 | 2.1 |
| Humidity (wt.%) | max. 2 | max. 2 | max. 3 | max. 2 |
| Parameter | Norit 1240 | WG12 | F300 | F830 |
|---|---|---|---|---|
| Height of filtration bed (cm) | 92 | 92 | 100 | 100 |
| Weight of the bed (g) | 1127.4 | 907.5 | 1418.3 | 1350.8 |
| Avg. flow through the column (mL·min−1) | 203.14 | 208.86 | 205.94 | 207.94 |
| Avg. filtration velocity (m·h−1) | 6.207 | 6.382 | 6.293 | 6.354 |
| Bed contact time (min) | 8.89 | 8.65 | 9.53 | 9.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barloková, D.; Ilavský, J.; Sedláková, J.; Matis, A. Removal of Humic Substances from Water with Granular Activated Carbons. Eng. Proc. 2023, 57, 22. https://doi.org/10.3390/engproc2023057022
Barloková D, Ilavský J, Sedláková J, Matis A. Removal of Humic Substances from Water with Granular Activated Carbons. Engineering Proceedings. 2023; 57(1):22. https://doi.org/10.3390/engproc2023057022
Chicago/Turabian StyleBarloková, Danka, Ján Ilavský, Jana Sedláková, and Alena Matis. 2023. "Removal of Humic Substances from Water with Granular Activated Carbons" Engineering Proceedings 57, no. 1: 22. https://doi.org/10.3390/engproc2023057022
APA StyleBarloková, D., Ilavský, J., Sedláková, J., & Matis, A. (2023). Removal of Humic Substances from Water with Granular Activated Carbons. Engineering Proceedings, 57(1), 22. https://doi.org/10.3390/engproc2023057022






