How Low Can It Go? ATR-FTIR Characterization of Compounds Isolated from Ginger at the Nanogram Level †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure
2.2. Experiment 1
2.3. Experiment 2
2.4. Data Analysis and Interpretation of FTIR Spectra
3. Results and Discussion
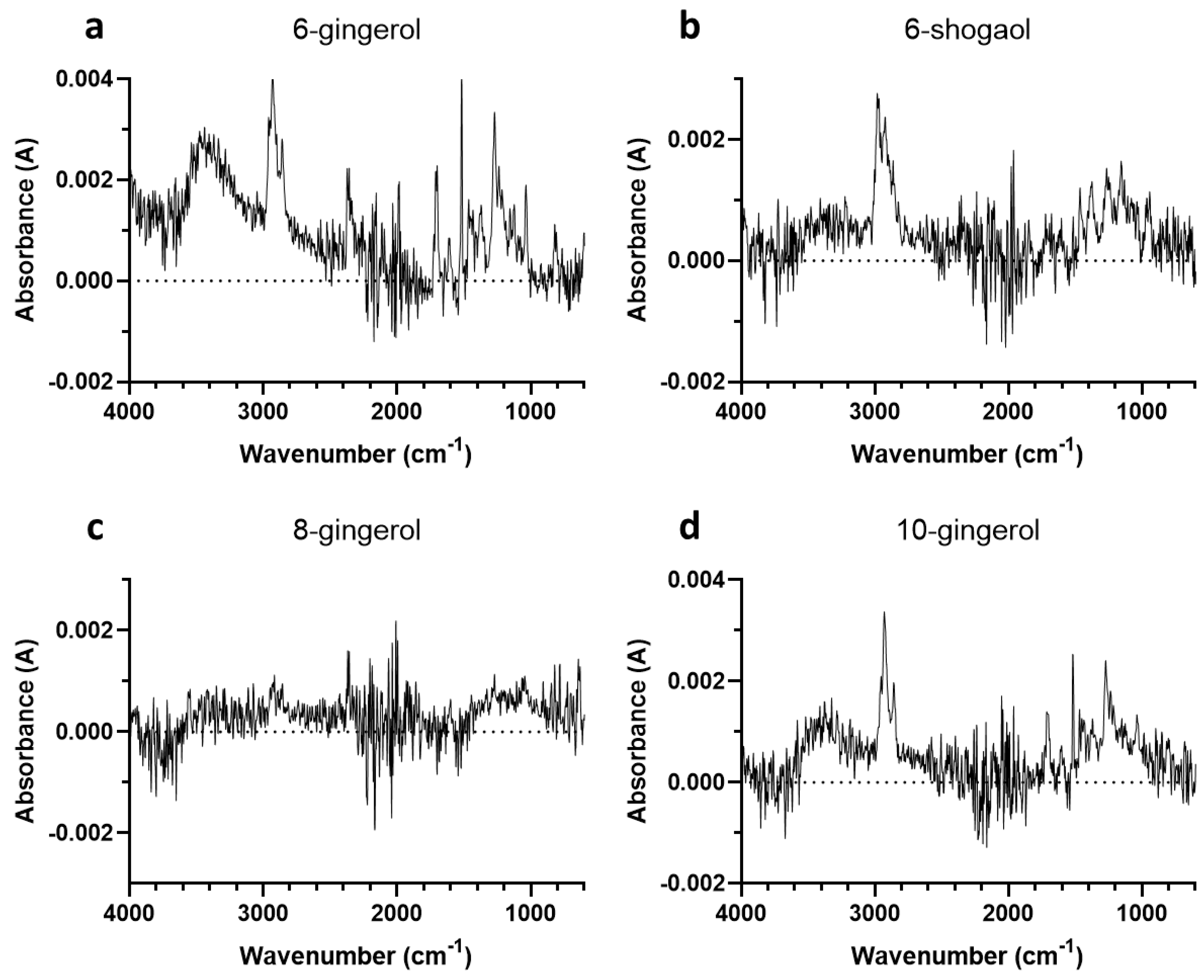
3.1. First Experiment
3.1.1. Determination of Mass of 6-Gingerol and Related Compounds
3.1.2. FTIR Spectra
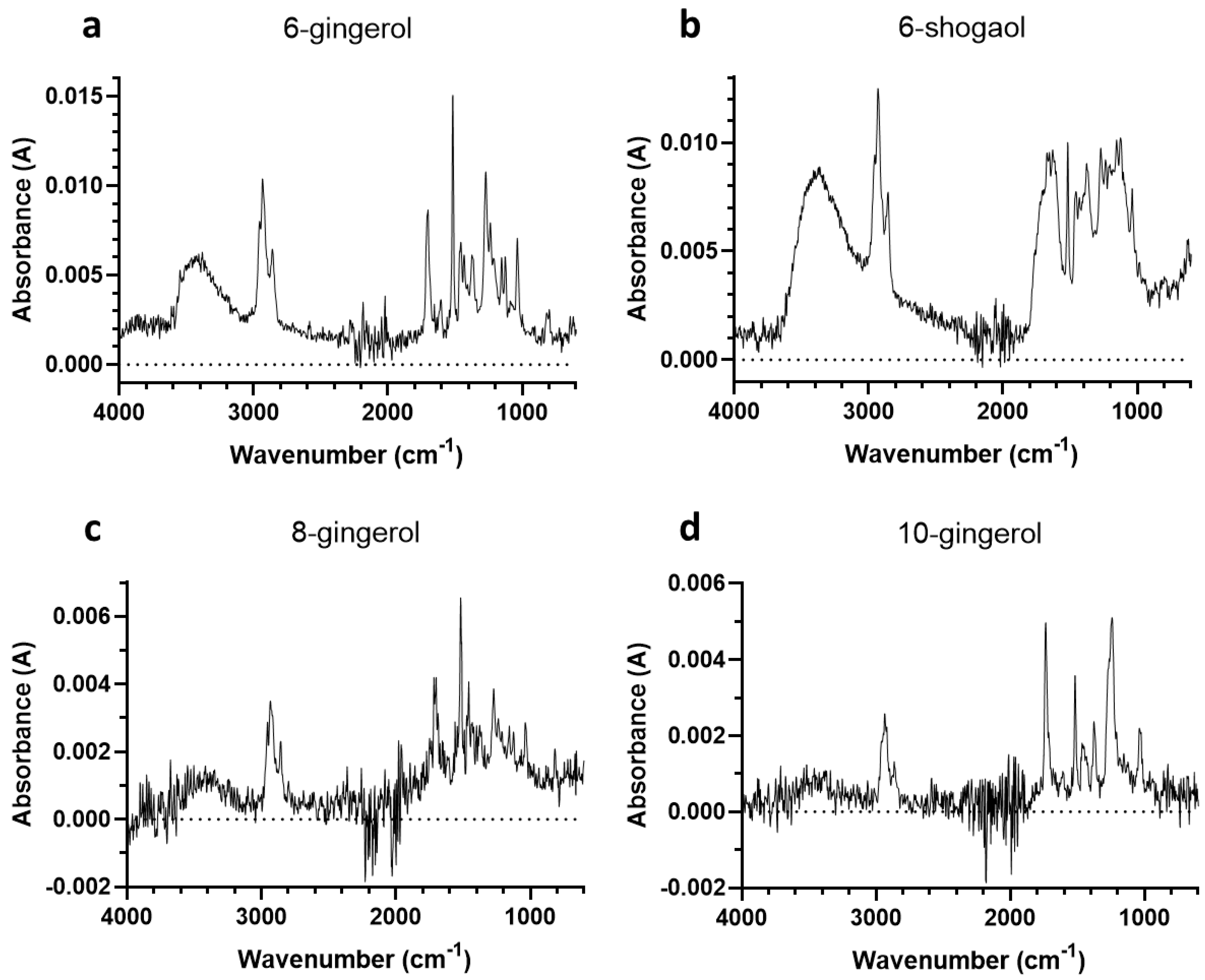
3.2. Second Experiment
3.2.1. Determination of Mass of 6-Gingerol and Related Compounds
3.2.2. FTIR Spectra
3.3. Assignment of FTIR Spectra
3.4. Interpretation of FTIR Spectra
- One or more O-H groups (less visible in 6-shogaol and 8-gingerol).
- One or more CH3 groups.
- One or more CH2 groups.
- A CH2/CH3 ratio of ≥3, indicating the presence of at least 3 CH2 groups.
- A methoxy group, as indicated by the presence of an aromatic ether and saturated ether group.
- A methoxy (-O-CH3) group based on the CH3 absorbance at ~2845 cm−1 (note that this was not observed in 8-gingerol, possibly due to the small sample mass; however, the main methoxy feature above was seen in 8-gingerol);
- One or more benzene rings;
- Tentative: a phenol group;
- Tentative: a ketone with a saturated C-C(=O)-C structure;
- A secondary alcohol.
- An aliphatic (i.e., non-conjugated) ketone;
- A 1,2,4-trisubstituted benzene (note that this was not seen in 10-gingerol).
- A disubstituted, trans alkene;
- A conjugated alkene.
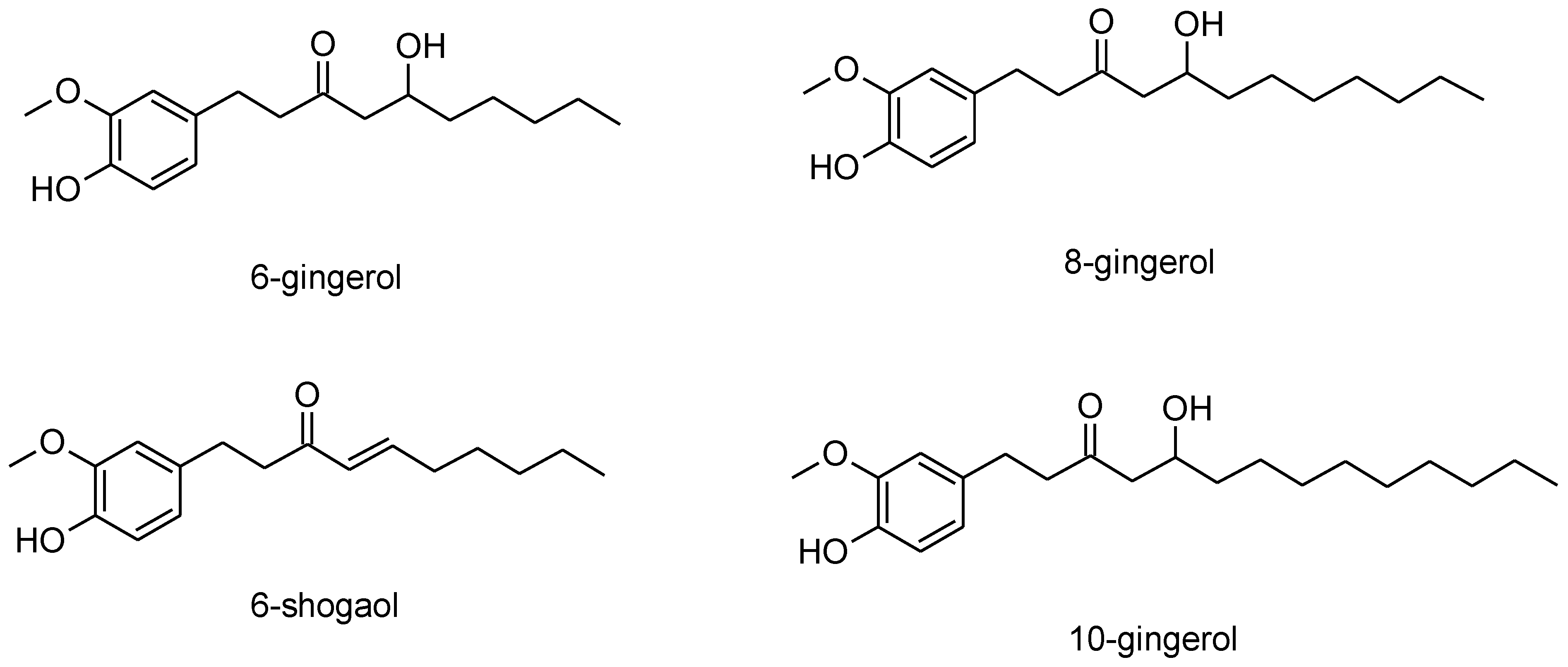
3.5. Synthesis of the Derived Information
- Begin with the benzene ring;
- At the 1, 2 and 4 positions, add the following:
- o
- A phenol group;
- o
- A methoxy group (alternatively, the benzene group could have two alkane chains, and the methoxy group could be located on one of them; placing the methoxy group on the benzene ring would require some familiarity with other similar natural structures such as vanillin, or more detailed structural information using a different analytical technique);
- o
- Possibly an alkane chain (of unknown length, but at least six carbons long if this is the only alkane chain).
- Add a secondary alcohol group at the second carbon or further down the alkane chain;
- Add a ketone group at the third carbon or further down the alkane chain;
- Assuming that only one alkane chain was attached to the benzene group, at least three CH2 groups would be required on this chain (i.e., excluding the C-OH and C=O carbons) to satisfy the ≥3CH2/CH3 ratio. Consequently, the alkane chain would have to be at least five carbons in length. Additionally, no alkene groups would be included in the chain, as the FTIR spectra did not show any alkene bonds aside from the benzene ring.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, R.B. Infrared Spectra and Organic Chemistry. Rev. Sci. Instrum. 1936, 7, 265–271. [Google Scholar] [CrossRef]
- Johnson, J.B.; Walsh, K.B.; Naiker, M.; Ameer, K. The Use of Infrared Spectroscopy for the Quantification of Bioactive Compounds in Food: A Review. Molecules 2023, 28, 3215. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Mani, J.S.; White, S.; Brown, P.; Naiker, M. Quantitative profiling of gingerol and its derivatives in Australian ginger. J. Food Compos. Anal. 2021, 104, 104190. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; White, S.; Brown, P.; Naiker, M. Pungent and volatile constituents of dried Australian ginger. Curr. Res. Food Sci. 2021, 4, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Sigma Aldrich. IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html (accessed on 31 March 2023).
- Raja, P.M.; Barron, A.R. 4.2: IR Spectroscopy. In Physical Methods in Chemistry and Nano Science; OpenStax CNX: Houston, TX, USA, 2023. [Google Scholar]
- Smith, B. IR Spectral Interpretation Workshop. Spectroscopy 2015, 30, 16–23. [Google Scholar]
- Smith, B. The CO bond III: Ethers by a knockout. Spectroscopy 2017, 32, 22–26. [Google Scholar]
- Degen, I.A. Detection of the Methoxyl Group by Infrared Spectroscopy. Appl. Spectrosc. 1968, 22, 164–166. [Google Scholar] [CrossRef]
| Compound | Equivalent Mass Used (ng) |
|---|---|
| 6-gingerol | 184 |
| 6-shogaol | 24 |
| 8-gingerol | 40 |
| 10-gingerol | 70 |
| Compound | Equivalent Mass Used (ng) |
|---|---|
| 6-gingerol | 598 |
| 6-shogaol | 76 |
| 8-gingerol | 90 |
| 10-gingerol | 53 |
| Assigned Bond | 6-Gingerol | 6-Shogaol | 8-Gingerol | 10-Gingerol | ||||
|---|---|---|---|---|---|---|---|---|
| Equiv. mass (ng) | 184 | 598 | 24 | 76 | 40 | 90 | 70 | 53 |
| Experiment | Expt 1 | Expt 2 | Expt 1 | Expt 2 | Expt 1 # | Expt 2 | Expt 1 | Expt 2 |
| O-H stretch (alcohol, intermolecular bonded) | 3439 br | 3381 br | 3364 b | ~3377 br w | ||||
| ~3146 br w | ||||||||
| CH3 asymmetric stretch | 2958 sh | 2954 sh | 2977 | 2952 sh | 2954 sh | 2952 sh w | 2958 sh w | |
| CH2 asymmetric stretch | 2927 | 2934 | 2923 w | 2929 | 2915 w | 2932 | 2927 | 2936 |
| CH2 symmetric stretch | 2857 | 2859 | 2861 sh w | 2855 | 2855 | 2857 | 2864 w | |
| -O-CH3 symmetric stretch | 2845 sh w | 2845 sh w | 2826 sh w | 2845 sh w | ||||
| 2798 w | ||||||||
| 1715 sh | 1734 | |||||||
| C=O stretch, aliphatic ketone | 1703 | 1701 | 1699 | ~1705 br | 1713 sh w | |||
| C=C stretch, disubstituted (trans) | ~1664 | |||||||
| C=C stretch, conjugated alkene | 1627 | |||||||
| 1608 w | 1604 w | 1598 w | ~1604 w | ~1600 w | ~1608 w | |||
| 1559 sh | ||||||||
| 1540 w | ||||||||
| C=C stretch, benzene ring | 1515 | 1515 | 1513 w | 1517 | 1517 | 1517 | 1517 | |
| 1488 w | ||||||||
| C-CH3 asymmetric bend | 1462 w | 1458 | 1462 w | 1458 | 1458 | 1466 w | 1463 w | |
| -CH2- scissors? | 1449 w | 1449 w | ||||||
| 1431 w | 1433 w | 1431 w | ~1433 w | |||||
| 1398 | 1404 w | |||||||
| O-H in-plane bend (phenol)? | 1369 w | 1373 | 1373 w | 1377 | 1375 | 1369 w | 1375 | |
| C-O stretch, aromatic ether | 1270 | 1270 | 1264 w | 1272 | 1270 w | 1272 | 1270 | 1270 sh w |
| C-O-H stretch (phenol) | 1235 w | 1235 w | 1235 w | 1235 w | 1235 w | 1241 | ||
| 1214 w | 1210 sh w | 1210 w | 1212 w | 1208 sh w | ||||
| Saturated C-C-C stretch, ketone? | 1154 w | 1152 | 1154 w | 1152 | 1155 w | 1148 w | 1152 w | |
| C-OH stretch, 2° alcohol? | 1121 w | 1126 | 1124 | 1124 w | ||||
| C-O stretch, saturated ether | 1033 | 1035 | 1037 | 1035 | 1037 w | 1037 | ||
| C=C bend, disubstituted (trans) | ~963 w | |||||||
| 1,2,4-trisubstituted benzene | 818 | 814 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.B.; Batley, R.J.; Mani, J.S.; Naiker, M. How Low Can It Go? ATR-FTIR Characterization of Compounds Isolated from Ginger at the Nanogram Level. Eng. Proc. 2023, 56, 80. https://doi.org/10.3390/ASEC2023-15407
Johnson JB, Batley RJ, Mani JS, Naiker M. How Low Can It Go? ATR-FTIR Characterization of Compounds Isolated from Ginger at the Nanogram Level. Engineering Proceedings. 2023; 56(1):80. https://doi.org/10.3390/ASEC2023-15407
Chicago/Turabian StyleJohnson, Joel B., Ryan J. Batley, Janice S. Mani, and Mani Naiker. 2023. "How Low Can It Go? ATR-FTIR Characterization of Compounds Isolated from Ginger at the Nanogram Level" Engineering Proceedings 56, no. 1: 80. https://doi.org/10.3390/ASEC2023-15407
APA StyleJohnson, J. B., Batley, R. J., Mani, J. S., & Naiker, M. (2023). How Low Can It Go? ATR-FTIR Characterization of Compounds Isolated from Ginger at the Nanogram Level. Engineering Proceedings, 56(1), 80. https://doi.org/10.3390/ASEC2023-15407








