Abstract
In this work, we describe the fabrication of paper-based aptasensing devices for ampicillin determination that rely on the salt-induced aggregation of gold nanoparticles (AuNPs) in the presence of the target. Circular paper-based devices were created on paper via pen-plotting (using water-repellent ink to create hydrophobic barriers) and modified with NaCl. The sample was incubated with an ampicillin aptamer and AuNPs and was added to the assay zones of the paper-based devices. In the absence of ampicillin, the aptamer prevented the aggregation of the AuNPs, and the assay zones remained red. When ampicillin was present, it selectively bound with the aptamer and the AuNP aggregate, producing a purple color. The color of the assay zones was monitored via a smartphone, and the color graduation was related to the ampicillin concentration in the sample. Different experimental parameters (type of paper, concentration of reagents) were investigated, and the analytical features of the method for the determination of ampicillin were established.
1. Introduction
Antibiotics are being widely used for the prevention and treatment of bacterial infections in farming [1]. The extensive use, abuse or misuse of antibiotics in food-producing animals may lead to residues finding their way into animal-derived foods (such as meat, milk and dairy products) and the natural environment. As a result of this process, long-term exposure to antibiotic residues can increase antibiotic resistance and potentially cause health problems to human consumers [2,3,4].
Therefore, it is important to develop low-cost, simple, fast, selective and sensitive detection technologies for the determination of antibiotics in different matrices. The “golden standard” for the identification and determination of antibiotics are liquid chromatography approaches, often hyphenated to mass spectrometry (LC-MS), which offer unambiguous confirmation, high sensitivity and multi-analyte capabilities [5,6]. However, these methodologies require expensive and bulky equipment, well-trained staff and extensive sample pretreatment. On the other hand, immunoassays (based on the use of antibodies for target recognition) [7] and biosensors [8,9] offer distinct advantages over LC-MS in terms of portability, rapidity, cost and, more importantly, scope for on-site and field assays.
Aptamers are gaining increasing popularity for antibiotic detection, serving as bioreceptors in biosensors and bioassays [10,11,12,13]. Aptamers, also named “artificial enzymes”, are short oligonucleotide sequences that exhibit binding affinity towards selected target analytes and have some distinct important advantages over antibodies. Paper-based analytical devices (PADs) have attracted increased attention in the last fifteen years, as they are inexpensive, portable sensing platforms for different analytical applications, using cellulose paper as a functional support [14,15,16,17,18,19].
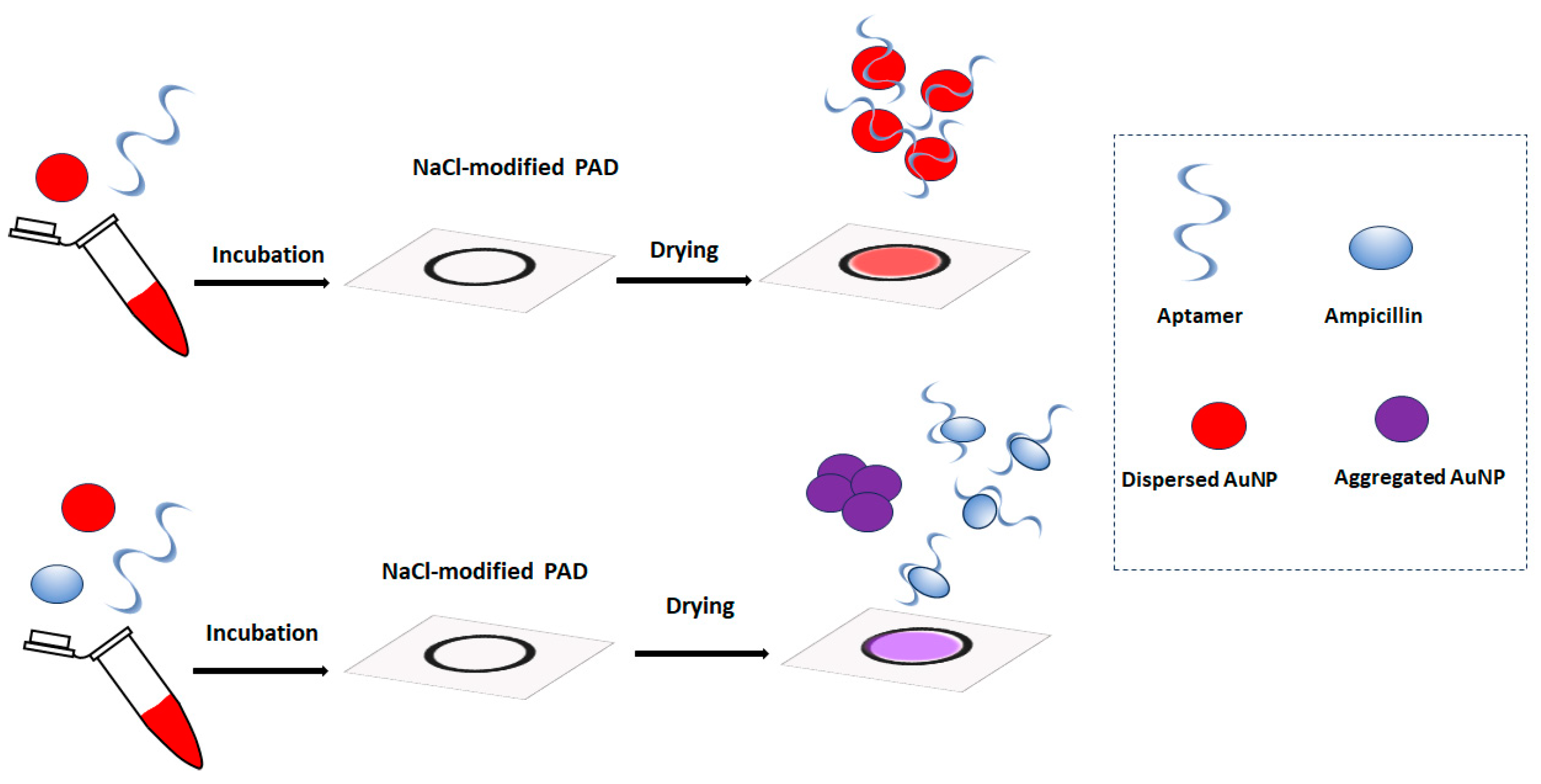
In this work, we describe a new type of paper-based aptasensing devices for ampicillin determination that rely on the salt-induced aggregation of gold nanoparticles (AuNPs) in the presence of the target [20]. To the best of our knowledge, this is the first report of a simple paper-based device for ampicillin detection. Although lateral flow assays on functional nitrocellulose strips have been reported for ampicillin detection [21], these devices are complex to fabricate and require modified capture probes. The principle of the assay proposed in this work is illustrated in Figure 1. Initially, paper-based devices are patterned on paper via pen-plotting using hydrophobic ink and modified with NaCl. The sample is incubated with an ampicillin aptamer and AuNPs and added to the paper-based device. In the absence of ampicillin, the aptamer prevents the aggregation of the AuNPs, and the devices are colored red. When ampicillin is present, it selectively binds with the aptamer and the unprotected AuNP aggregate, producing a purple color. The color of the paper-based devices is monitored via a smartphone, and the color graduation is related to the ampicillin concentration in the sample.
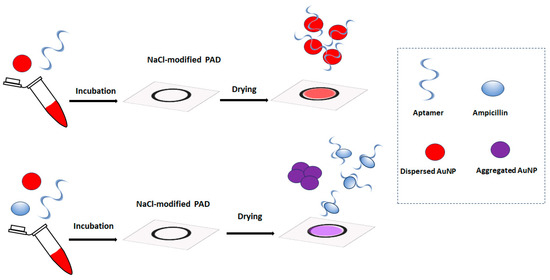
Figure 1.
The principle of the paper-based colorimetric aptamer assay for ampicillin with salt-induced aggregation of AuNPs.
2. Experimental
2.1. Reagents and Materials
All chemicals used for the preparation of stock and standard solutions were of analytical reagent grade and purchased from Sigma-Aldrich. The ampicillin aptamer was purchased from Integrated DNA Technology (IDT) (USA), and its sequence was 5′-TGG GGG TTG AGG CTA AGC CGA C-3′.
2.2. Experimental Protocol
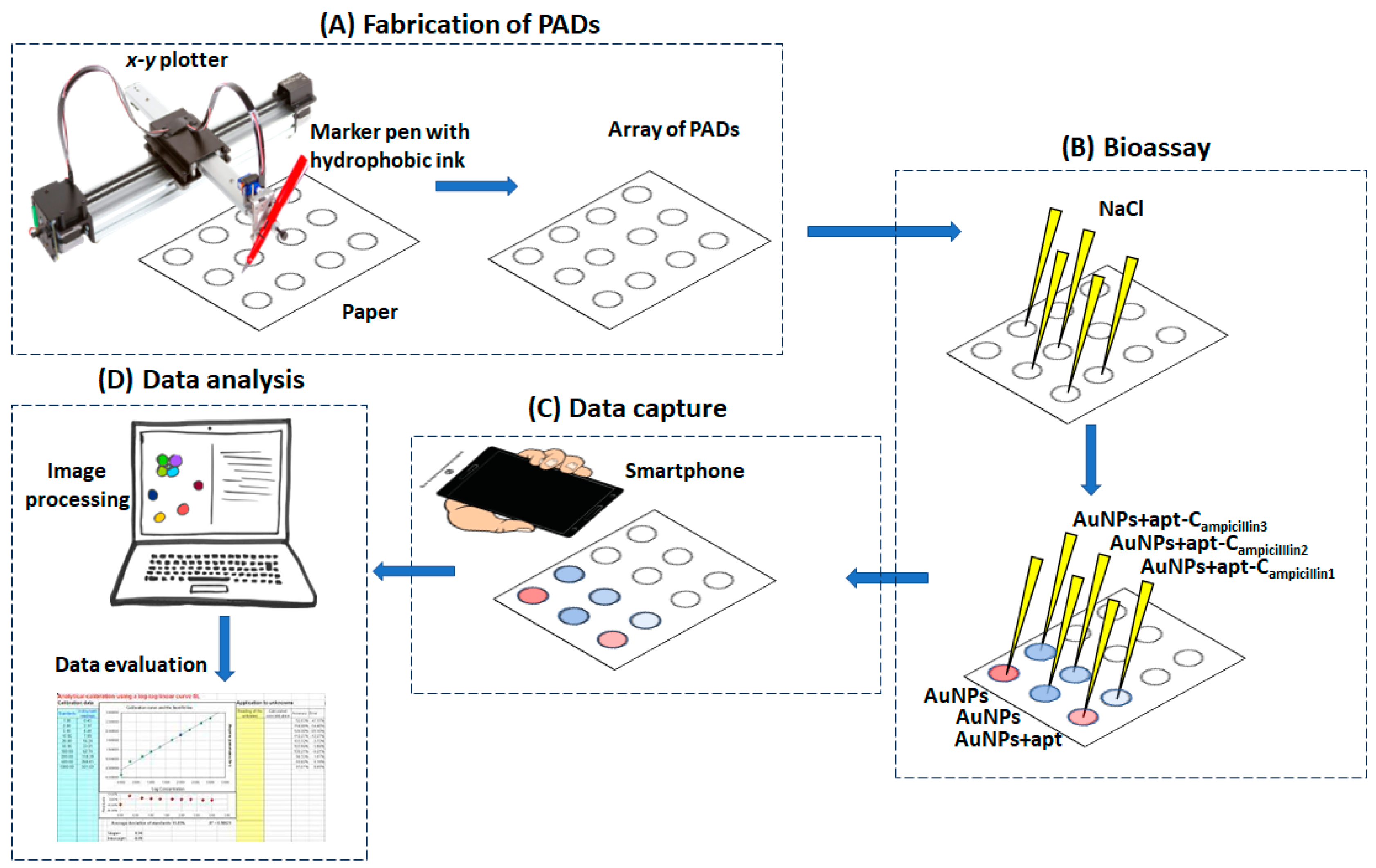
The experimental protocol is schematically illustrated in Figure 2.
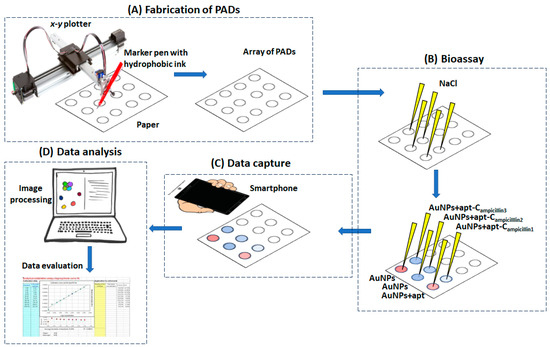
Figure 2.
The experimental protocol for the fabrication of the PADs, the bioassay and data capture, analysis and evaluation.
The paper-based devices were plotted using an AxiDraw desktop x-y plotter (Evil Mad Science LLC, Sunnyvale, CA, USA). The control software was the AxiDraw extension for Inkscape operated via the open-access software Inkscape (Inkscape Project, https://inkscape.org/about/, accessed on 24 October 2023). The paper support was Whatman grade 42 filter paper, and a hydrophobic marker pen (Edding 780 0.8 mm tip thickness (black)) was used for plotting.
The paper based-devices were modified with salt by adding 8 μL of a 1.0 M NaCl solution, and the devices were left to dry.
A 13.3 mM AuNPs solution was incubated with 5 μM of aptamer solution for 1 h. Then, an ampicillin standard in the range 50–750 μg L−1 was added to the aptamer/AuNPs solution and further incubated for 30 min. Finally, 8 μL of the aptamer/AuNPs/ampicillin solution was added to the salt-modified paper substrate and left to dry at room temperature.
Upon drying, the image of the paper-based devices was captured using a smartphone (Samsung A12), and the image file was transferred to InkScape. The scanned image was filtered using the fluorescent preset filter, making the red color more vibrant. The “color picker” tool was implemented to measure the H-value, using the HSV (hue, saturation, value) color space. The H-value for each measurement was subtracted from the H-value of the blank experiment; higher blank-subtracted H-values corresponded to stronger “purple” color intensity. Data plotting and reporting were performed in Excel.
3. Results and Discussion
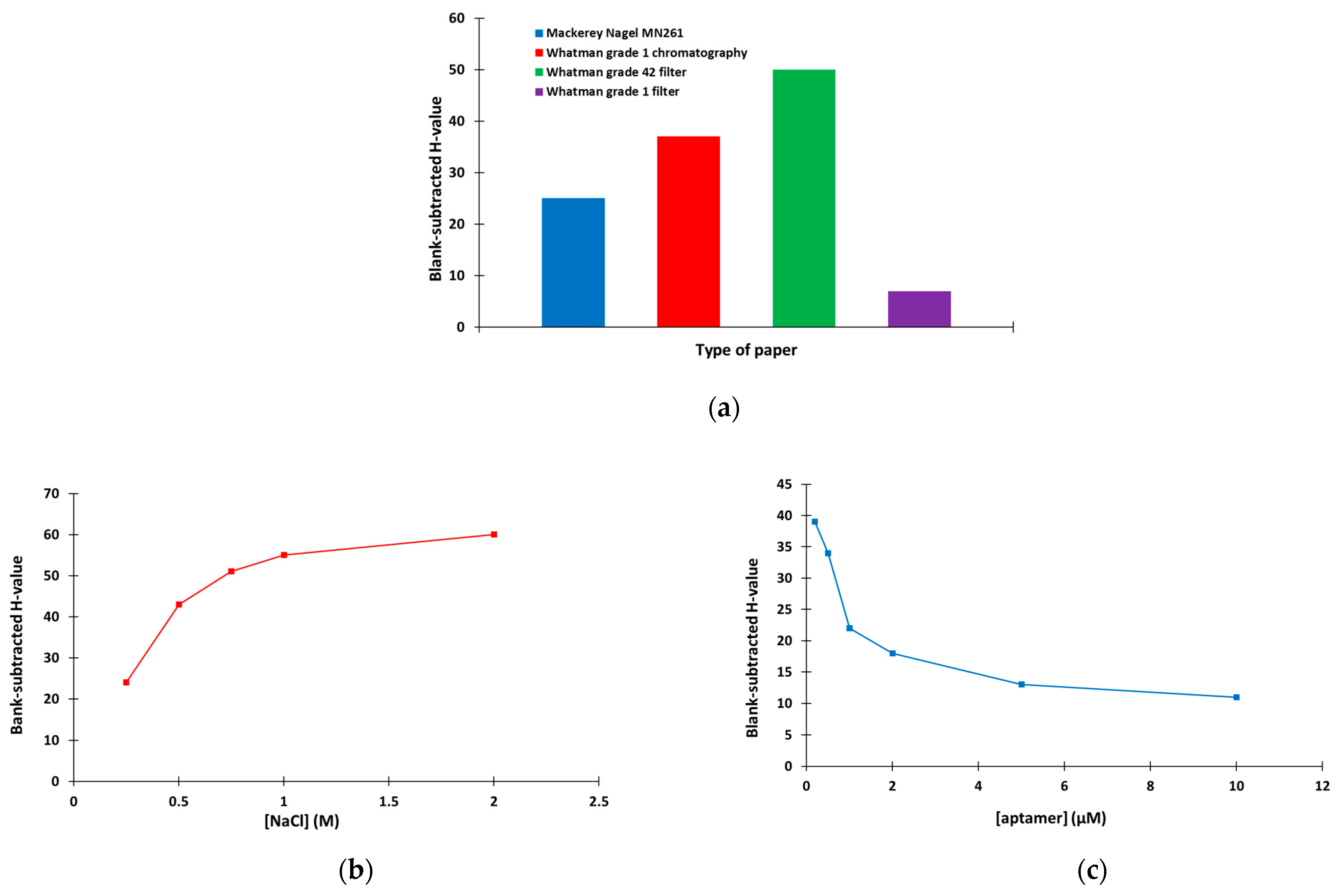
The method optimization involved study of the type of the paper support, the NaCl concentration used to modify the devices and the aptamer concentration. Four types of paper support were studied (namely Mackerey Nagel MN261 chromatography paper, Whatman grade 1 chromatography paper, Whatman grade 42 filter paper and Whatman grade 1 filter paper) in terms of the aggregation capacity of AuNPs in the presence of NaCl (expressed in terms of the H-value). As illustrated in Figure 3a, the strongest aggregation was obtained with the Whatman grade 42 filter paper, which was used for further experiments. Next, the NaCl concentration that induced the most efficient aggregation of AuNPs was investigated. As shown in Figure 3b, NaCl concentrations ≥ 1 M were sufficient to induce the maximum aggregation of AuNPs, and 1 M NaCl was selected. Finally, the aptamer concentration that was required to protect the AuNPs from the salt-induced aggregation was selected. Figure 3c indicates that the protection of AuNPs from aggregation increased as the aptamer concentration increased (reflected in the decreasing H-values); 5 μM of aptamer was selected for the rest of this work.
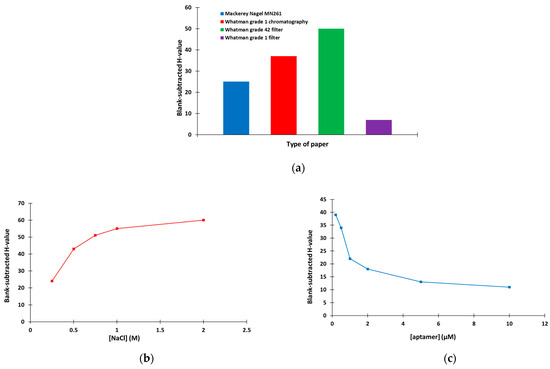
Figure 3.
Selection of (a) the type of paper (8 μL of 13.3 mM AuNPs + 8 μL of 1 M ΝaCl), (b) the concentration of NaCl (8 μL of 13.3 mM AuNPs + 8 μL of ΝaCl at Whatman grade 42 filter paper) and (c) the concentration of aptamer (different concentrations of aptamer diluted in 10 mM of phosphate buffer (pH 7.4) containing 2 mM of MgCl2 was incubated with 13.3 mM AuNPs for 1 h and applied to the paper-based device modified with 1 M NaCl).
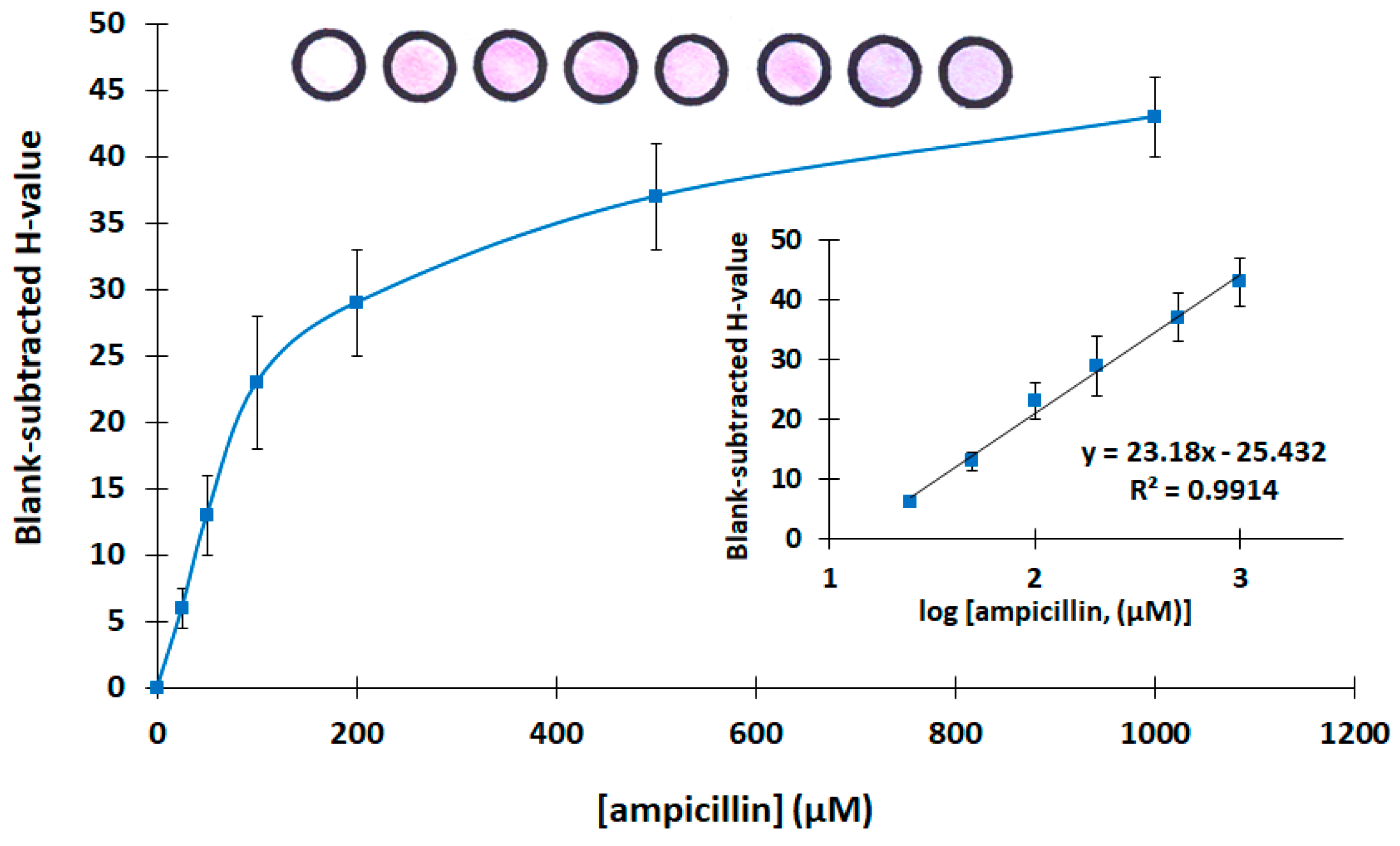
Then, the analytical features of the assay were evaluated. Calibration for ampicillin was carried out in the concentration range 0–1000 μg L−1. The calibration plot is shown in Figure 4, while photographs of the respective paper-based devices with different target concentrations and the linear-log calibration plot are shown as inserts. Each calibration point is the mean of three assays, and the error bars in Figure 4 represent the standard deviation of the three assays. The limit of detection was calculated as 10 μg L−1 using the formula LOD = 3.3 × sb/S (where sb is the standard deviation of the intercept of the calibration plot, and S is the slope of the linear part of the calibration plot). The mean relative standard deviation across the calibration range (including six calibration points) was 16.9%.
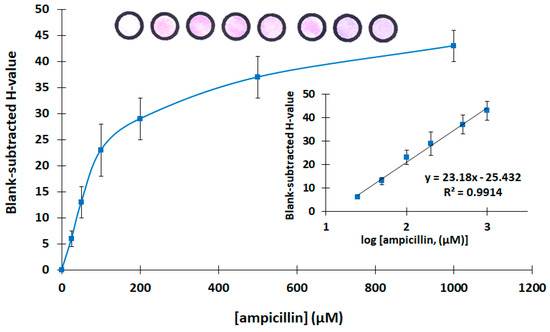
Figure 4.
Calibration of ampicillin (the linear-log transformation of the calibration plot and photographs of the paper-based sensors at the different ampicillin concentrations are shown as inserts. The photograph at the left is a device with buffer only).
4. Conclusions and Prospects
In this work, a colorimetric paper-based aptasensing approach for the assay of ampicillin was developed. The method for the fabrication of the paper-based devices (pen-plotting with hydrophobic ink) is fast, low-cost and convenient, and the protocol of the aptamer-based assay is simple, without the requirement for labels or other probes. Instrument-free quantitative analysis can be performed using only a smartphone as a recording device. Work is in progress to improve the limit of detection and implement the assay using real samples.
Author Contributions
Conceptualization, A.E. and C.K.; methodology, A.E., C.K. and D.S.; validation, D.S.; investigation, D.S.; data curation, A.E. and D.S.; writing—original draft preparation, A.E. and D.S.; writing—review and editing, A.E. and D.S.; supervision, A.E.; project administration, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 101007299.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Peris-Vicente, J.; Peris-García, E.; Albiol-Chiva, J.; Durgbanshi, A.; Ochoa-Aranda, E.; Carda-Broch, S.; Bose, D.; Esteve-Romero, J. Liquid chromatography, a valuable tool in the determination of antibiotics in biological, food and environmental samples. Microchem. J. 2022, 177, 107309. [Google Scholar] [CrossRef]
- Li, F.; Luo, J.; Zhu, B.; Liu, Z. Pretreatment Methods for the Determination of Antibiotics Residues in Food Samples and Detected by Liquid Chromatography Coupled with Mass Spectrometry Detectors: A Review. J. Chromatogr. Sci. 2022, 60, 991–1003. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Abu Bakr Shabbir, M.; Cheng, G.; Yuan, Z. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Sun, C.; Li, Y. Recent advances in biosensors for antibiotic detection: Selectivity and signal amplification with nanomaterials. Food Chem. 2021, 361, 130109. [Google Scholar] [CrossRef]
- Hong, J.; Su, M.; Zhao, K.; Zhou, Y.; Wang, J.; Zhou, S.-F.; Lin, X. A Minireview for Recent Development of Nanomaterial-Based Detection of Antibiotics. Biosensors 2023, 13, 327. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.C.; Bacher, G.; Bhand, S. Recent Advances in Aptamer- Based Biosensors for Detection of Antibiotic Residues. Aptamers Synth. Antibodies 2013, 2, 43–54. [Google Scholar]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors 2022, 22, 3684. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Song, L.; Gao, Y.; Wu, K.; Guo, R.; Chen, R.; Zhen, J.; Pan, L. Aptamer Sensors for the Detection of Antibiotic Residues A Mini-Review. Toxics 2023, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Tribhuwan Singh, A.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.T.; Hou, C.Y.; Wang, Y.N.; Fu, L.M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sens. Actuators B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Fu, L.M.; Wang, Y.N. Detection methods and applications of microfluidic paper-based analytical devices. Trends Anal. Chem. (TrAC) 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Lim, H.; Turab Jafry, A.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in Paper-Based Analytical Devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef]
- Noviana, E.; Carrão, D.B.; Pratiwi, R.; Henry, C.S. Emerging applications of paper-based analytical devices for drug analysis: A review. Anal. Chim. Acta 2020, 1116, 70–90. [Google Scholar] [CrossRef]
- Nooranian, S.; Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Kazemi Oskuee, R. Biosensors based on aptamer-conjugated gold nanoparticles: A review. Biotechnol. Appl. Biochem. 2022, 69, 1517–1534. [Google Scholar] [CrossRef]
- Lin, H.; Fang, F.; Zang, J.; Su, J.; Tian, Q.; Kumar Kankala, R.; Lin, X. A Fluorescent Sensor-Assisted Paper-Based Competitive Lateral Flow Immunoassay for the Rapid and Sensitive Detection of Ampicillin in Hospital Wastewater. Micromachines 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).