Abstract
This study discusses the design, development, and construction of a low-cost optical multisensor system. The light sources in the proposed system are lanthanide(III) complexes emitting light in the near-infrared spectral region. Through the ability to adjust the source wavelength, this measuring platform can be used for a variety of practical tasks. The feasibility of the developed devices is demonstrated for the analysis of milk samples.
1. Introduction
In recent decades, various industries have recognized the importance of quality control of their production at all stages [1,2]. Analytical techniques, aiming to assess physical and chemical properties of target products, must meet the growing demands of any modern industry: quick analysis, use without highly qualified personnel, low price, and reasonable maintenance costs.
Optical sensors are one of the most popular components of new portable analytical devices, developed for food analysis [3,4,5], environmental monitoring [6,7], disease diagnosis [8,9], etc. A wide range of available sensors allows the creation of a custom-designed measurement system for a particular task and medium to be analyzed. To create a reliable device, it is necessary to choose a light source and other optical parts, find a suitable measuring geometry, and test the system on standard samples or on a large number of real samples.
Sometimes, it is beneficial to use a synergy of several cross-sensitive sensors and to combine them into a single optical multisensor system (OMS) [10]. However, in this case, a suitable chemometric algorithm should also be developed to process a complex unresolved signal and extract necessary information from the response of system.
In this study, we report a novel measurement technique for OMS based on a multiband light source with tunable intensity and wavelength range. Nd(III) and Yb(III) complexes that have luminescence emission bands in the near-infrared (NIR) spectral region [11] were used as a light source. The developed OMS was applied for the quantitative determination of fat in milk and for the detection of milk adulteration with urea. The proposed approach has great potential for a wide range of practical applications in dairy and other industries.
2. Complexes
Ln(III) complexes ([Ln(tta)3(dppn)], Ln = Nd (1), Yb (2); Htta = thenoyltrifluoroacetone; dppn = 3,6-di(2-pyridyl)pyridazine) were synthesized [12] in order to obtain light sources for OMSs. Lanthanide complexes were chosen based on the following reasons: 1. Predictability of NIR emission energy. 2. Nd(III) and Yb(III) complexes are the most popular NIR emitters for working with biological objects, because their photoemission is within the transparency window of most biological objects (700–1100 nm). 3. The luminescence of Ln(III) is not sensitive to oxygen, which allows it to work without sample degassing. 4. Tris(beta-diketonate) heteroleptic Ln(III) complexes are air-, moisture-, and photostable.
The selected molecular emitters meet the following requirements: the absorption spectrum does not overlap with the emission spectrum; the excitation radiation at 365 nm fits into the absorption maximum; and the emission is intense enough to be used as a light source in the solid phase. This allows their use as a multiband light source in the design of OMSs.
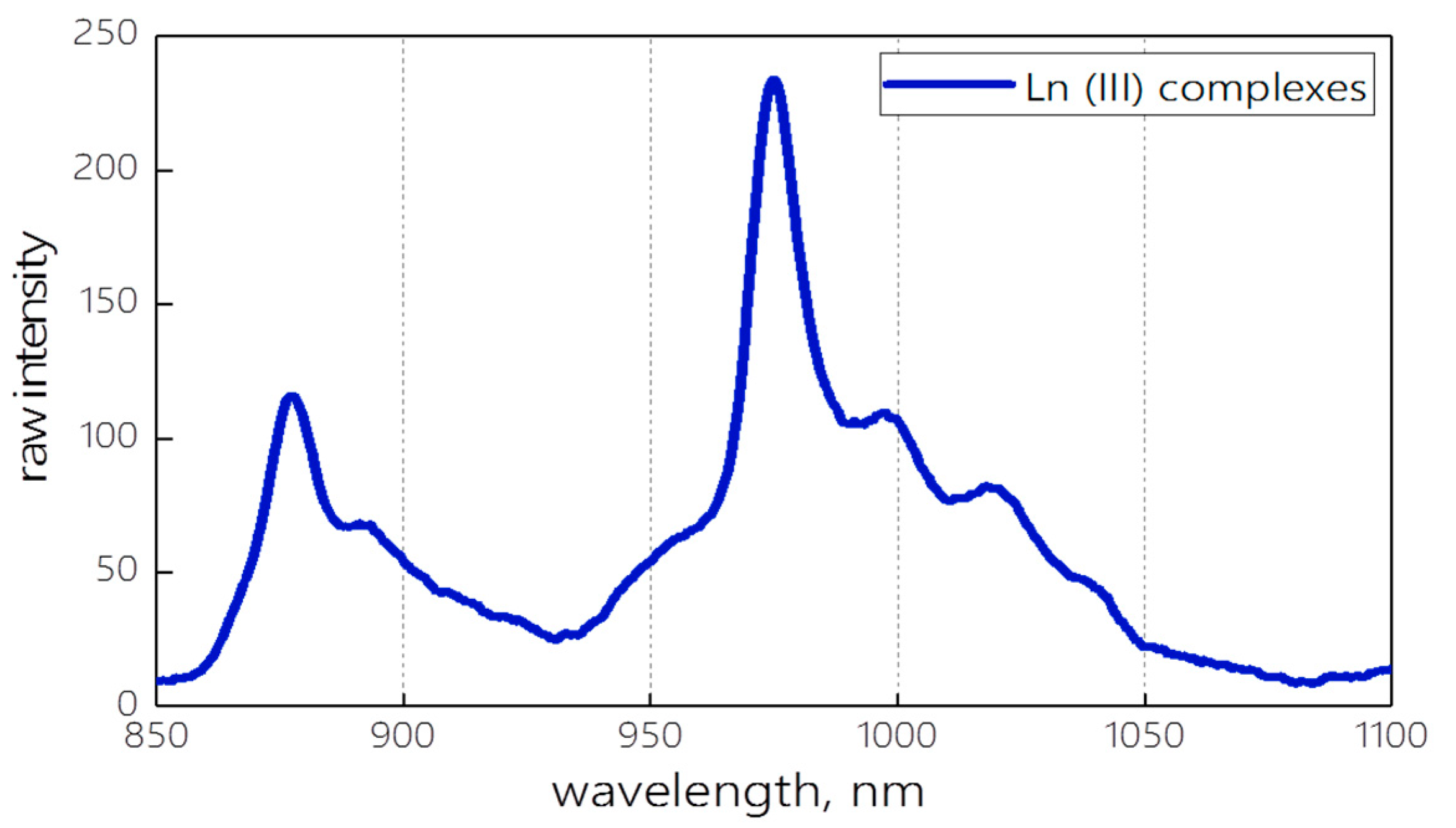
The resulting spectrum of the mixture of Nd(III) and Yb(III) complexes upon excitation with a UV flashlight is shown in Figure 1. The mixture of the complexes emits in the region of 850–1150 nm with two emission maxima at 877 and 975 nm.
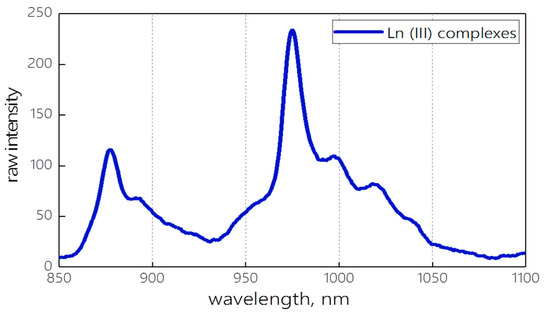
Figure 1.
The emission spectrum of Ln(III) complexes’ mixture.
3. Design of OMS
The optical setup was constructed as follows. A mixture of lanthanide(III) complexes (10 mg of the unit of 1 complex and 5 mg of the unit of 2 complex) was placed on a glass substrate under the sample. Since the light intensity of lanthanide(III) complexes is low, the use of a liquid sample was not appropriate. Therefore, we proposed the analysis of a dried drop of a sample solution placed on the surface of a thin cover glass. A UV flashlight with λexct = 365 nm was used as the excitation light of molecular emitters. The advantages of the UV flashlight are its price and higher power compared with the laser diode. Furthermore, the UV flashlight excites a stronger emission of the lanthanide(III) complexes.
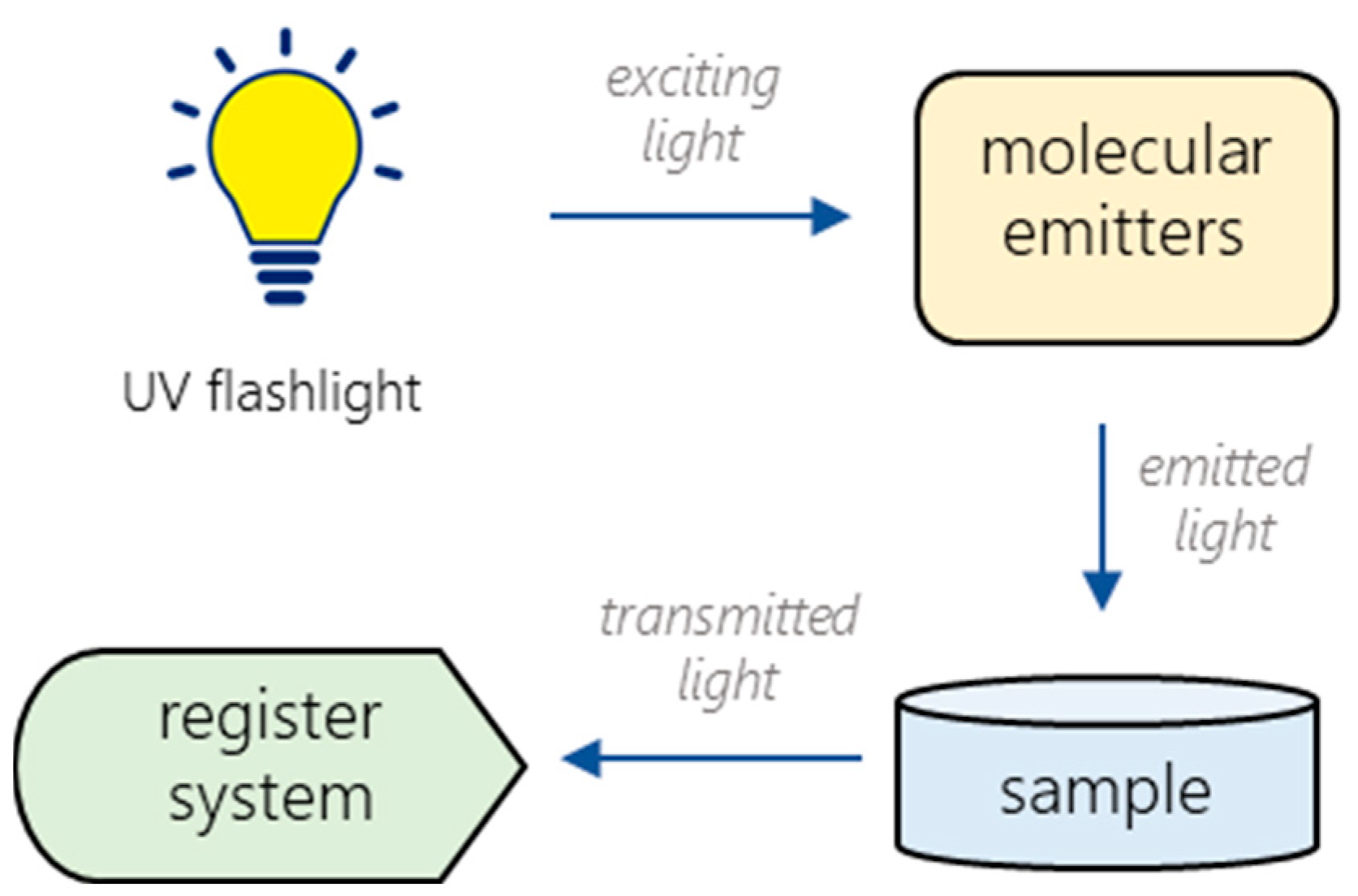
According to the experimental setup (Figure 2), the light from the UV flashlight excites the emission of the lanthanide(III) complexes. Then, the emitted light passes through a sample and registers with the special optical system (STS-NIR miniature spectrometer by Ocean Optics (Largo, FL, USA)). The optimal device geometry, the required amount, and the placement of the sample solution were experimentally chosen for the prototype to obtain a stable and reproducible analytical signal.
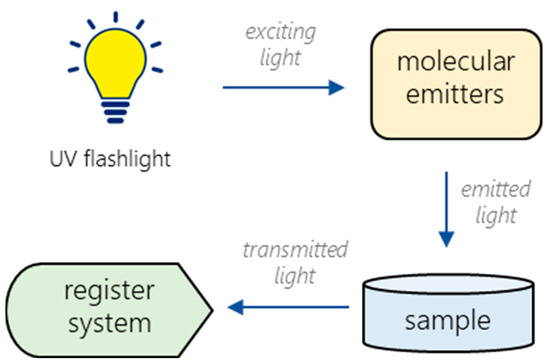
Figure 2.
Block diagram of the experimental setup.
4. Practical Application of the OMS
Milk is a mass-market product and one of the most popular targets for adulteration. Urea that is present in natural milk in concentrations from 180 to 400 mg/L with a possible maximum of 700 mg/L is often added to simulate the presence of a high amount of protein in the diluted milk [13]. The methods for the determination of urea, such as Raman spectroscopy, ion chromatography, or liquid chromatography–mass spectrometry, are expensive, labor-intensive, and require a high level of analytical expertise. Urea has a weak absorption band in the spectral region from 800 to 1100 nm, where the second overtone of –NH2 symmetric stretching is observed [14]. The OMS consisting of two Ln(III) complexes as a light source was examined for the qualitative determination of urea in milk.
To build a classification model, a calibration set was prepared from milk (with fat content of 2.5%) with the addition from 0 to 0.09 g of urea. The calibration set consisted of ten samples (10 mL each) with urea concentrations of 0, 100, 300, 500, 700, 1000, 3000, 5000, 7000, and 9000 mg/L. The concentration range was chosen to ensure that five samples contained urea below or equal to the possible level in raw milk (700 mg/L). The other five samples contained urea above the possible level and were accepted as adulterated. Partial least-squares–discriminant analysis (PLS-DA) was used to classify “adulterated” and “non-adulterated” samples. The PLS-DA model (Table 1) demonstrates a good analytical performance for classification between the two sample groups.

Table 1.
PLS-DA modeling and validation statistics.
Another practical task, addressed in this study, was related to the determination of fat content in milk. Fat is one of the important nutrient components in milk that define its quality, and its content should be controlled at all stages of production. To assess the performance of the developed OMS prototype in the quantification of fat in milk, a set of five homogenized milk samples with varying fat content was prepared. The fat content of the calibration set ranged from 2.5 to 3.3% in 0.2% increments. A partial least-squares (PLS) regression model for fat content determination was trained on the spectra in the 850–1100 nm region and the resulting PLS model achieved a relatively high prediction accuracy: the root-mean-square error of cross-validation was 0.09% and R2 = 0.94.
5. Conclusions
The combination of lanthanide(III) and Ir(III) or Cu(I) complexes that have been described in our previous works [15,16] can be used as a multichannel light source in the visible and NIR spectral range with a tunable intensity and wavelength range and can be adopted for the particular analytical task by selecting the appropriate wavelength region. The proposed approach for OMS construction allows for reducing the analysis time and does not require a complex sample preparation. The application of chemometrics provides a high accuracy of analysis, comparable with that of full-featured spectrometers.
Author Contributions
A.S.: Methodology, Investigation, Formal Analysis, Writing—Original draft. A.B.: Conceptualization, Writing—Review and Editing. A.P.: Investigation. V.K.: Investigation. E.B.: Investigation, Data Analysis, Writing—Review and Editing. E.G.: Supervision, Writing—Review and Editing. D.K.: Supervision, Conceptualization, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
Surkova A. acknowledges the financial support from the President’s grant #MK-2192.2021.4 (construction and optimization of optical multisensor systems, data analysis). Bogomolov A. acknowledges the financial support from the Ministry of Science and Higher Education of the Russian Federation (theme No. FSSE-2023-0003) as part of the state task of the Samara State Technical University (conceptualization, experimental design).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skibsted, E.; Engelsen, S.B. Spectroscopy for process analytical technology (PAT). Spectrosc. Lett. 2017, 3, 188e197. [Google Scholar] [CrossRef]
- Rolinger, L.; Rüdt, M.; Hubbuch, J. A critical review of recent trends, and a future perspective of optical spectroscopy as PAT in biopharmaceutical downstream processing. Anal. Bioanal. Chem. 2020, 412, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Pampuri, A.; Tugnolo, A.; Giovenzana, V.; Casson, A.; Guidetti, R.; Beghi, R. Design of cost-effective LED based prototypes for the evaluation of grape (Vitis vinifera L.) ripeness. Comput. Electron. Agric. 2021, 189, 106381. [Google Scholar] [CrossRef]
- Civelli, R.; Giovenzana, V.; Beghi, R.; Naldi, E.; Guidetti, R.; Oberti, R. A simplified, light emitting diode (LED) based, modular system to be used for the rapid evaluation of fruit and vegetable quality: Development and validation on dye solutions. Sensors 2015, 15, 22705–22723. [Google Scholar] [CrossRef] [PubMed]
- Lastra-Mejias, M.; Villa-Martinez, A.; Izquierdo, M.; Aroca-Santos, R.; Cancilla, J.C.; Torrecilla, J.S. Combination of LEDs and cognitive modeling to quantify sheep cheese whey in watercourses. Talanta 2019, 203, 290–296. [Google Scholar] [CrossRef]
- Yeh, P.; Yeh, N.; Lee, C.-H.; Ding, T.-J. Applications of LEDs in optical sensors and chemical sensing device for detection of biochemicals, heavy metals, and environmental nutrients. Renew. Sustain. Energy Rev. 2017, 75, 461–468. [Google Scholar] [CrossRef]
- Sohrabi, H.; Hemmati, A.; Majidi, M.R.; Eyvazi, S.; Jahanban-Esfahlan, A.; Baradaran, B. Recent advances on portable sensing and biosensing assays applied for detection of main chemical and biological pollutant agents in water samples: A critical review. Trends Anal. Chem. 2021, 143, 116344. [Google Scholar] [CrossRef]
- Negi, S.; Mittal, P.; Kumar, B.; Juneja, P.K. Organic LED based light sensor for detection of ovarian cancer. Microelectron. Eng. 2019, 218, 111154. [Google Scholar] [CrossRef]
- Costa-Fernandez, J.M.; García, C.M.; Soldado, A. Optical (Bio)Sensors in medical diagnosis. Biomaterials 2023, 4, 297–316. [Google Scholar] [CrossRef]
- Bogomolov, A. Developing multisensory approach to the optical spectral analysis. Sensors 2021, 21, 3541. [Google Scholar] [CrossRef] [PubMed]
- Surkova, A.; Bogomolov, A.; Paderina, A.; Khistiaeva, V.; Boichenko, E.; Grachova, E.; Kirsanov, D. Optical multisensor system based on lanthanide(III) complexes as near-infrared light sources for analysis of milk. Chemosensors 2022, 10, 288. [Google Scholar] [CrossRef]
- Khistiaeva, V.V.; Melnikov, A.S.; Slavova, S.O.; Sizov, V.V.; Starova, G.L.; Koshevoy, I.O.; Grachova, E.V. Heteroleptic -diketonate Ln(III) complexes decorated with pyridyl substituted pyridazine ligands: Synthesis, structure and luminescence properties. Inorg. Chem. Front. 2018, 5, 3015. [Google Scholar] [CrossRef]
- Ezhilan, M.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Babu, K.J.; Krishnan, U.M.; Rayappan, J.B.B. Design and development of electrochemical biosensor for the simultaneous detection of melamine and urea in adulterated milk samples. Sens. Actuators B Chem. 2017, 238, 1283–1292. [Google Scholar] [CrossRef]
- Siesler, H.W.; Kawata, S.; Heise, H.M.; Ozaki, Y. Near-Infrared Spectroscopy: Principles, Instruments, Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Gitlina, A.Y.; Surkova, A.; Ivonina, M.V.; Sizov, V.V.; Petrovskii, S.; Legin, A.; Starova, G.L..; Koshevoy, I.O.; Grachova, E.V.; Kirsanov, D.O. Cyclometalated Ir(III) complexes as tuneable multiband light sources for optical multisensor systems: Feasibility study. Dyes Pigm. 2020, 180, 108428. [Google Scholar] [CrossRef]
- Surkova, A.A.; Paderina, A.V.; Legin, A.V.; Grachova, E.V.; Kirsanov, D.O. Cu(I)-based molecular emitters for quantification of fluoride and phosphate in surface waters. Measurement 2021, 184, 109976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).