Abstract
The degradation of lignocellulose in biogas processes has been focused on the inoculant microorganisms involved, with a view to gaining a deeper understanding in order to improve lignocellulose degradation. The maximum volumetric biogas yield (12.17 L/L) was achieved with the inoculum used in experiment “B”, containing 400 g of digestate from the bioreactor along with 400 g of rumen fluid. The highest concentration of methane in biogas was obtained from the same inoculum composition (63.2 ± 1.5%). The second largest volumetric yield of 8.41 ± 0.45 L/L biogas was achieved in experiment “C,” where digestates were used as the main inoculum. Accordingly, in this case, the volumetric yield of biogas was 8.41 ± 0.45 L/L. The composition of rumen fluid and digestate increased biogas production from the same amount of alfalfa leaves by 30.9%.
Keywords:
anaerobic inoculum; bacteria; feedstock; anaerobic digestion; alfalfa; biomass; biomethane 1. Introduction
Alfalfa leaves (AL) are an excellent feedstock as biogas production material for anaerobic co-digestion (AD) because of their organic solids content of more than 20%. However, they also have a high concentration of fibers and lignocellulose, components which introduce susceptibility to layering, making this feedstock problematic to digest in CSTR bioreactors. Lignocellulosic biomass has a huge potential to be used as a feedstock for the sustainable production of fuels and chemicals through fermentation. Today, plant substrates, also entitled lignocellulosic biomass, are seen as one of the most promising materials to replace fossil energy resources in the production of fuels and chemicals with reduced GHGs emissions [1].
The influence of inoculum used for anaerobic lignocellulosic biomass treatment in every specific case varies. In cellulolytic rumen bacteria, highly active cellulolytic and hemicellulolytic enzymes are combined in extracellular multienzyme complexes (cellulosomes) [2]. Recent research related to the degradation of lignocellulose in biogas processes has had a strong focus on the microorganisms involved, with the aim of further understanding and improving degradation. These studies have, e.g., evaluated the whole bacterial and archaea community by analyzing the 16 rRNA genes [3]. Improving the performance of the microbial strains for efficient conversion of sugars from complex substrates (hydrolysates produced from lignocellulosic biomass) is an important question to be solved to support the large-scale implementation of these bioprocesses. Researchers investigated the degradation of straw and cellulose during batch cultivation using material from different full-scale biogas plants as the inoculum source [4]. The results showed similar biogas yields but differences in the degradation rate, as well as a correlation between degradation rate and the composition of the cellulose-degrading community. Employing a combination of two or more microbial species for bioprocessing biomass into biogas remains an underrated strategy to increase processing efficiency. Revolutionizing biotechnological biomass usage, a cutting-edge approach that involves synergizing multiple microbial species for bioprocessing may enhance overall process efficiency. This co-cultivation approach can potentially alleviate some of the problems associated with lignocellulose biomass use. The general idea of this concept is to take advantage of the specialized ability of two or more organisms and create a synergistic effect. Since multiple strains are used in a single process, a broader variation in beneficial characteristics can be selected. Optimization of a co-cultivation process could then be performed by selecting the right strains to be combined, instead of engineering one do-it-all strain [2].
The addition of rumen fluid to the anaerobic digestion process can significantly enhance biogas production by providing a diverse range of microorganisms that possess the necessary enzymatic activity to break down complex lignocellulosic materials. The optimal range of rumen fluid addition varies depending on the type of feedstock and operational conditions of the biogas plant, with the ideal range being between 25 and 50%. However, it is important to carefully monitor the process and avoid overloading the system with rumen fluid, as this can lead to unwanted process disruptions [5].
The aim of the work was to investigate the influence of rumen anaerobic bacteria inoculum on biogas yield and quality from alfalfa biomass.
2. Materials and Methods
The influence of dairy rumen fluid inoculum selected for anaerobic treatment on the organic fraction of alfalfa leaves was studied in this work. Dairy rumen fluid was taken from a dairy farm in southwestern Lithuania. The rumen fluid was packaged in an airtight container of 15 L and stored at 37.0 ± 0.2 °C to be protected from environmental influences until the start of the experiment. The transportation period from the collection of rumen fluid to the start of the experiment took 2 h. Prior to the commencement of the experiment, the dairy rumen fluid was filtered through a 0.5 mm stainless steel mesh.
The chemical analysis of the feedstock was carried out on alfalfa biomass composition content. Dry organic matter (VS) was performed according to LST EN 13039:2012, using the gravimetric method.
A single-load biogas yield experiment was carried out on a biochemical methane potential test bench (BMP). The mesophilic temperature was maintained at 37.0 ± 0.2 °C during the experiment. To determine the potential biogas yield and production from alfalfa biomass, four separate BMP experiments were conducted with triplicate samples for each experiment set (Figure 1). As the feedstock composition in A, B, C sets, the same amount of alfalfa biomass was added—16 g. Reactor set “A” was inoculated with 800 g of rumen fluid (proportion 100%/0%), Reactor set “B” was inoculated with 400 g rumen fluid and 400 g digestate from laboratory bioreactor using wheat straw as a feedstock (proportion 50%/50%), and Reactor set “C” was loaded with 800 g digestate directly from the same bioreactor as mentioned in Reactor “B” (proportion 0%/100%). To evaluate residual methanogenic activity inoculum BMP, Reactor “D” was started without any alfalfa addition and it served as a negative control sample. The experiments were performed in triplicate to ensure experimental data reliability.
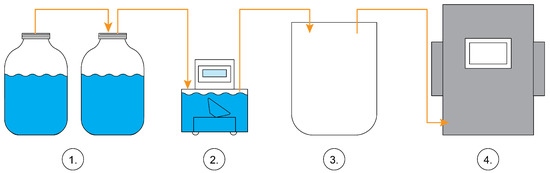
Figure 1.
Technological research scheme. (1) a set of BMP bioreactors; (2) Ritter Miligascounter; (3) Tedlar biogas bag; (4) Awite Bioenergie GmbH AwiFlex biogas analyzer.
Biogas volume from each bioreactor was monitored daily, and the concentrations of methane (CH4), carbon dioxide (CO2), and hydrogen sulphide (H2S) were monitored after the 35 days of experiment. The amount of gas formed was registered using RITTER MilliGascounters (2). The 20 L volume Tedlar PVF gas sampling bag (3) was used for biogas collection. The collected biogas was analyzed with an Awite Bioenergie GmbH AwiFlex (Germany) biogas analyzer (4).
3. Results and Discussion
The measured pH values of digestate and rumen fluid inoculants were, respectively, 7.3 and 6.1. The normal pH of dairy cow rumen fluid is typically between 6.0 and 7.0. However, it can fluctuate depending on the cow’s diet and feeding schedule [6]. The pH in the digestate typically varies from 6.5 to 8.2, [7] with optimal values for methane-producing anaerobic digestion being 6.8–7.2 [8].
The main feedstock for the experiment was crushed alfalfa leaves biomass, which had an evenly dry and homogeneous state at total solids (TS) content of 64.3% and concentration of 91.9%. The total and volatile solids content of alfalfa leaves can vary depending on factors such as the stage of growth, weather conditions, and location.
In the present study, the total solids and volatile solids tests were conducted for both the digestate and rumen fluid. The digestate and rumen fluid inoculants had low total solids concentrations in this experiment, with a respective concentration of 4% and 1.8%. The volatile solids content in these inoculants was observed to be high, with respective values of 98.2% and 97.4%. It is important to note that volatile and total solids experiments were conducted for sieved digestate and rumen fluid.
The results of the BMP experiment indicated that the highest volumetric biogas yield of 12.17 ± 0.62 L/L was achieved in test B, where a combination of rumen fluid and digestate was used as an inoculum for alfalfa leaves. The second highest volumetric biogas yield of 8.41 ± 0.45 L/L was obtained in test C, where bioreactor digestate was used as inoculum for alfalfa leaves. These findings suggest that the increase in biogas yield was due to the presence of highly active cellulolytic and hemicellulolytic enzymes, which are combined in extracellular multienzyme complexes known as cellulosomes [9].
Volumetric biogas yields from experiments A and D were the lowest. The lowest gain in biogas was from experiment “A” with rumen fluid and alfalfa biomass, which was only 1.14 L/L ± 0.17 L/L. The volume of gained biogas from experiment “A” was too low to analyze its composition. In experiment “A”, liquid rumen fluid was used as an inoculum and was utilized with alfalfa addition to assess its methanogenic activity. The lowest volumetric yield of biogas came from the digestate (0.35 ± 0.08 L/L), as it did not contain additional alfalfa leaves as biomass.
The concentration of methane in the biogas was also dependent on the inoculum used for the research. It is necessary to mention that only experiments B and C gained enough biogas to analyze with the Awite biogas analyzer. The highest concentration of methane was gained in experiment B (63.2 ± 1.5%). Biogas gained from the digestate (experiment C) had a lower concentration of methane, at 54.6 ± 1.1%. This finding agrees with the conclusions from the experiment conducted by Zheng et al., which demonstrated that a ratio of 1:5 of rumen microorganisms to biogas slurry yielded high methane production and content, thereby establishing it as the optimal ratio [10].
The biomethane yields from alfalfa volatile solids obtained in experiment B and experiment C were, respectively, 598 ± 8.3 and 357 ± 12.4 L/kg. The employment of digestate and ruminant inoculum resulted in a 32% increase in biomethane yield, in contrast to the C sample, where pure digestate was used as an inoculum. Experiment B yielded a biomethane yield of 668 ± 12.2 L/kg from the total solids of alfalfa, whereas experiment C produced a biomethane yield of 462 ± 18.3 L/kg.
Hakl et al. performed experimental research on alfalfa biomethane yield. In their experiment, approximately from 250 to 390 L CH4/kg from lucerne forage was obtained [11]. Comparison of research results suggest that optimizing the conditions of alfalfa digestion, such as feedstock characteristics, inoculum type, and operating conditions, leads to improved biomethane yields.
Experimental results of this research complement earlier (Nagler et al., 2019) research, in that the inclusion of rumen liquid enhances the degradation of complex lignocellulosic compounds by providing a diverse range of cellulolytic and hemicellulolytic microorganisms [12]. This leads to an increase in biogas production and improved process stability. The authors suggest that the addition of rumen liquid could be a simple and effective strategy to enhance the performance of lignocellulose-degrading biogas plants.
4. Conclusions
Through a series of laboratory BMP experiments, the effect of using rumen fluid from dairy cows as an inoculum on biogas production rate was studied. Experiment B, which inoculated rumen fluid and digestate, yielded the maximum volumetric biogas yield of 12.17 ± 0.62 L/L. The second largest volumetric yield of biogas was observed in experiment C, which used only digestate as inoculum, with a yield of 8.41 ± 0.45 L/L. The combination of rumen fluid and digestate in the inoculum for experiment B resulted in a 30.9% increase in biogas production from the same quantity of alfalfa biomass. The dairy rumen fluid-inoculated BMP experiment resulted in a methane concentration of 63.2 ± 1.5%, while the digestate-inoculated experiment yielded a slightly lower concentration of methane at 54.6 ± 1.1%. Therefore, the use of rumen fluid in combination with digestate increased the methane concentration by approximately 8.6% (63.2–54.6) in methane content in biogas. The biomethane yields from alfalfa volatile solids obtained in experiment B and experiment C were, respectively, 598 ± 8.3 and 357 ± 12.4 L/kg. The employment of digestate and ruminant inoculum resulted in a 32% increase in biomethane yield, compared to the C sample, where pure digestate was used as an inoculum. These findings suggest that utilizing a mixture of rumen fluid and digestate as the inoculum can significantly enhance the biogas production from alfalfa biomass.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ECP2023-14616/s1.
Author Contributions
Conceptualization, B.Ž. and K.N.; methodology, B.Ž., K.V. and E.B.; investigation, B.Ž. and M.R.; resources, B.Ž. and K.V.; writing—original draft preparation B.Ž. and K.V.; writing—review and editing, B.Ž., K.N. and K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledged the support received from Vytautas Magnus University, Agriculture Academy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindorfer, J.; Lettner, M.; Hesser, F.; Fazeni, K.; Rosenfeld, D.; Annevelink, B.; Mandl, M. Technical, Economic and Environmental Assessment of Biorefinery Concepts. 2019. Available online: https://www.ieabioenergy.com/wp-content/uploads/2019/07/TEE_assessment_report_final_20190704-1.pdf (accessed on 10 January 2023).
- Miron, J.; Ben-Ghedalia, D.; Morrison, M. Invited review: Adhesion mechanisms of rumen cellulolytic bacteria. J. Dairy Sci. 2001, 84, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, T.; Müller, B.; Schnürer, A. The microbial community Structure in industrial biogas plants influences the degradation rate of Straw and cellulose in batch tests. Biotechnol. Biofuels 2016, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Yamakawa, C.K.; van der Maas, L.; Dragone, G. New trends in bioprocesses for lignocellulosic biomass and CO2 utilization. Renew. Sustain. Energy Rev. 2021, 152, 111620. [Google Scholar] [CrossRef]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. Increasing Biogas Production Rate from Cattle Manure Using Rumen Fluid as Inoculums. Int. J. Sci. Eng. 2014, 6, 31–38. [Google Scholar] [CrossRef]
- Penner, G.B.; Beauchemin, K.A.; Mutsvangwa, T. Severity of ruminai acidosis in primiparous holstein cows during the periparturient period. J. Dairy Sci. 2007, 90, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Singh, S.P. Bioenergy conversion studies of organic fraction of MSW: Kinetic studies and gas yield-organic loading relationships for process optimisation. Bioresour. Technol. 2004, 95, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Lamed, R.; Himmel, M.E. The potential of cellulases and cellulosomes for cellulosic waste management. Curr. Opin. Biotechnol. 2007, 18, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Yang, F. Improving the Anaerobic Digestion of Switchgrass via Cofermentation of Rumen Microorganisms (Rumen Bacteria, Protozoa, and Fungi) and a Biogas Slurry. Energy Fuels 2019, 33, 1185–1195. [Google Scholar] [CrossRef]
- Hakl, J.; Fuksa, P.; Habart, J.; Šantrůček, J. The biogas production from lucerne biomass in relation to term of harvest. Plant Soil Environ. 2012, 58, 289–294. [Google Scholar] [CrossRef]
- Nagler, M.; Kozjek, K.; Etemadi, M.; Insam, H.; Podmirseg, S.M. Simple yet effective: Microbial and biotechnological benefits of rumen liquid addition to lignocellulose-degrading biogas plants. J. Biotechnol. 2019, 300, 1–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).