Synthesis and Application of Magnesium-Based Nanoparticles for the Photocatalytic Degradation of Methylene Blue in Aqueous Solutions: Optimization and Kinetic Modeling †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Methylene Blue Solution
2.3. Photocatalyst Synthesis
2.4. Characterization
2.5. Photocatalytic Degradation
2.5.1. Degradation Experiments
2.5.2. Statistical Analysis
3. Results and Discussion
3.1. UV-Vis DRS
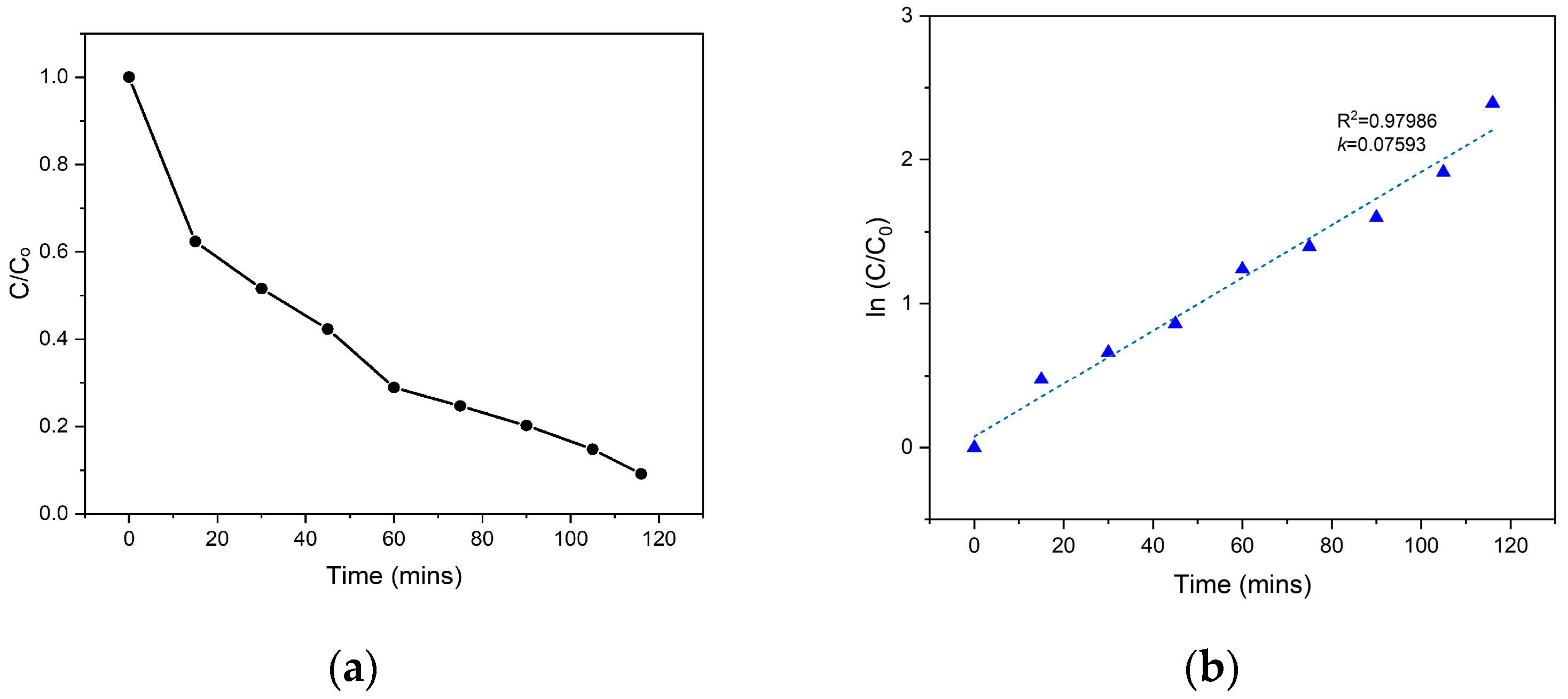
3.2. Optimization, Validation, and Kinetics
3.3. Effects of Operational Parameters
3.3.1. Effect of Dye Concentration
3.3.2. Effect of Catalyst Dose
3.3.3. Effect of pH
3.3.4. Effect of Time
3.4. Economic Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R. Soybean hulls as a low-cost biosorbent for removal of methylene blue contaminant. Environ. Prog. Sustain. Energy 2020, 39, e13328. [Google Scholar] [CrossRef]
- Ebi Ebi, O.; Falilat Taiwo, A.; Tunde Folorunsho, A. Kinetic Modelling of the Biosorption of Methylene Blue onto Wild Melon (Lagenariasphaerica). Am. J. Chem. Eng. 2018, 6, 126–134. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.A.H.; Aziz, K.H.H.; Omer, K.M.; Salih, Y.M.; Mustafa, F.; Rahman, K.O.; Mohammad, Y. Degradation of aqueous organic dye pollutants by heterogeneous photo-assisted Fenton-like process using natural mineral activator: Parameter optimization and degradation kinetics. IOP Conf. Ser. Earth Environ. Sci. 2021, 958, 012011. [Google Scholar] [CrossRef]
- Advanced Oxidation Processes (AOP)|Spartan. Spartan Water Treatment. Available online: https://spartanwatertreatment.com/advanced-oxidation-processes/ (accessed on 19 March 2023).
- Rao, K.G.; Ashok, C.H.; Rao, K.V.; Chakra, C.H.S. Structural properties of MgO Nanoparticles: Synthesized by Co-Precipitation Technique Structural properties of MgO Nanoparticles: Synthesized by Co-Precipitation Technique. Int. J. Sci. Res. 2014, 3, 43–46. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band Gap Narrowing and Widening of ZnO Nanostructures and Doped Materials. Nanoscale Res. Lett. 2015, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Almontasser, A.; Parveen, A.; Azam, A. Synthesis, Characterization and antibacterial activity of Magnesium Oxide (MgO) nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577, 012051. [Google Scholar] [CrossRef]
- Mensah, K.; Samy, M.; Ezz, H.; Elkady, M.; Shokry, H. Utilization of iron waste from steel industries in persulfate activation for effective degradation of dye solutions. J. Environ. Manag. 2022, 314, 115108. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Heydari, M.; Javanbakht, V. Photocatalytic and photo-fenton processes by magnetic nanophotocatalysts for efficient dye removal. J. Mater. Sci. Mater. Electron. 2021, 32, 5065–5081. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Alkaim, A.; Aljeboree, A.; Alrazaq, N.A.; Jaafer, S.; Hussein, F.; Lilo, A.J. Effect of pH on Adsorption and Photocatalytic Degradation Efficiency of Different Catalysts on Removal of Methylene Blue. Asian J. Chem. 2014, 26, 8445–8448. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. The photocatalytic degradation of Methyl Green in presence of Visible light with photoactive Ni 0.10:La 0.05:TiO2 nanocomposites. IOSR J. Appl. Chem. 2017, 10, 31–44. [Google Scholar] [CrossRef]
- Munawar, T.; Mukhtar, F.; Yasmeen, S.; Naveed-ur-Rehman, M.; Nadeem, M.S.; Riaz, M.; Mansoor, M.; Iqbal, F. Sunlight-induced photocatalytic degradation of various dyes and bacterial inactivation using CuO–MgO–ZnO nanocomposite. Environ. Sci. Pollut. Res. 2021, 28, 42243–42260. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, S.; Das, S. Glucose-mediated one-pot hydrothermal synthesis of hollow magnesium oxide-zinc oxide (MgO-ZnO) microspheres with enhanced natural sunlight photocatalytic activity. Environ. Sci. Pollut. Res. 2023, 30, 8512–8525. [Google Scholar] [CrossRef] [PubMed]
- Revathi, V.; Karthik, K. Microwave assisted CdO–ZnO–MgO nanocomposite and its photocatalytic and antibacterial studies. J. Mater. Sci. Mater. Electron. 2018, 29, 18519–18530. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngulube, K.F.; Nasr, M.; Fujii, M.; Abdelhaleem, A. Synthesis and Application of Magnesium-Based Nanoparticles for the Photocatalytic Degradation of Methylene Blue in Aqueous Solutions: Optimization and Kinetic Modeling. Eng. Proc. 2023, 37, 75. https://doi.org/10.3390/ECP2023-14636
Ngulube KF, Nasr M, Fujii M, Abdelhaleem A. Synthesis and Application of Magnesium-Based Nanoparticles for the Photocatalytic Degradation of Methylene Blue in Aqueous Solutions: Optimization and Kinetic Modeling. Engineering Proceedings. 2023; 37(1):75. https://doi.org/10.3390/ECP2023-14636
Chicago/Turabian StyleNgulube, Khumbolake Faith, Mahmoud Nasr, Manabu Fujii, and Amal Abdelhaleem. 2023. "Synthesis and Application of Magnesium-Based Nanoparticles for the Photocatalytic Degradation of Methylene Blue in Aqueous Solutions: Optimization and Kinetic Modeling" Engineering Proceedings 37, no. 1: 75. https://doi.org/10.3390/ECP2023-14636
APA StyleNgulube, K. F., Nasr, M., Fujii, M., & Abdelhaleem, A. (2023). Synthesis and Application of Magnesium-Based Nanoparticles for the Photocatalytic Degradation of Methylene Blue in Aqueous Solutions: Optimization and Kinetic Modeling. Engineering Proceedings, 37(1), 75. https://doi.org/10.3390/ECP2023-14636







