Photocatalytic Degradation of Malathion Using Hydroxyapatite Derived from Chanos chanos and Pangasius dory Bones †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Hydroxyapatite
2.3. Photocatalytic Degradation of Malathion
3. Results
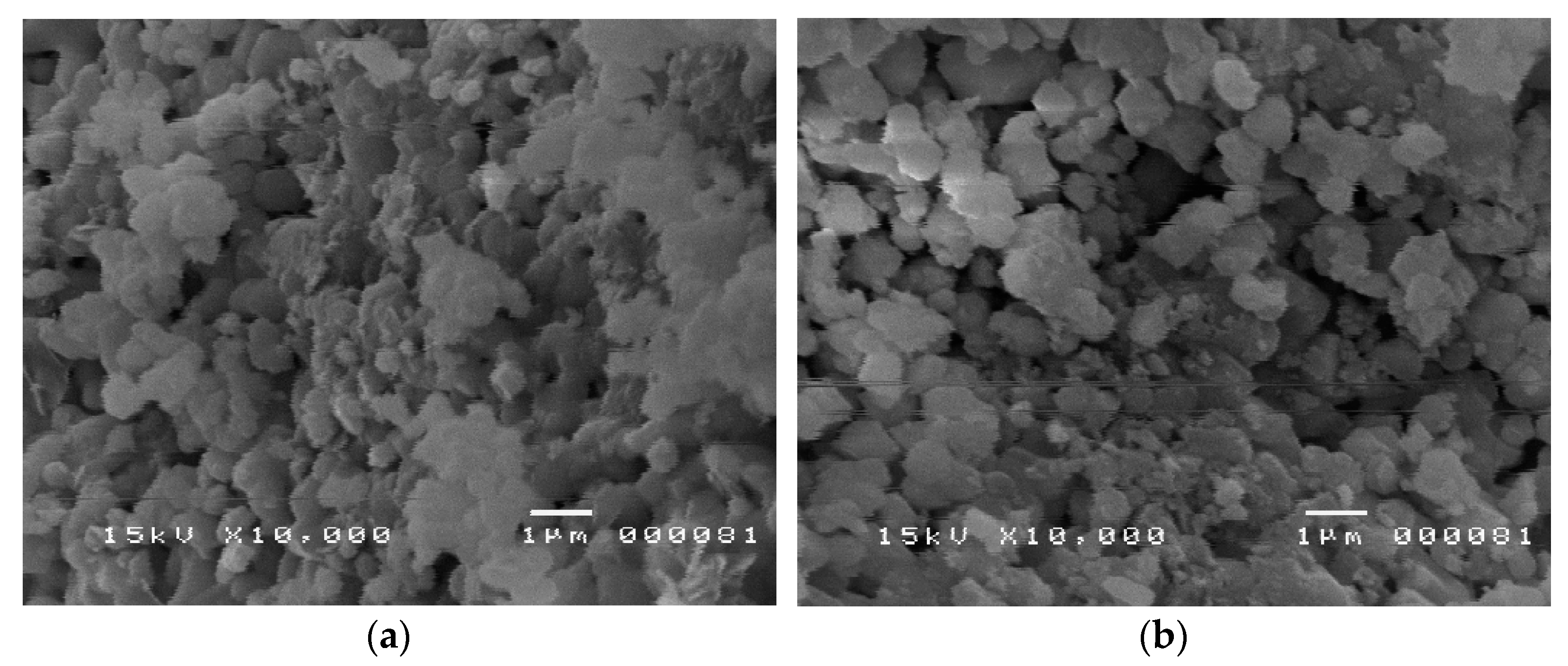
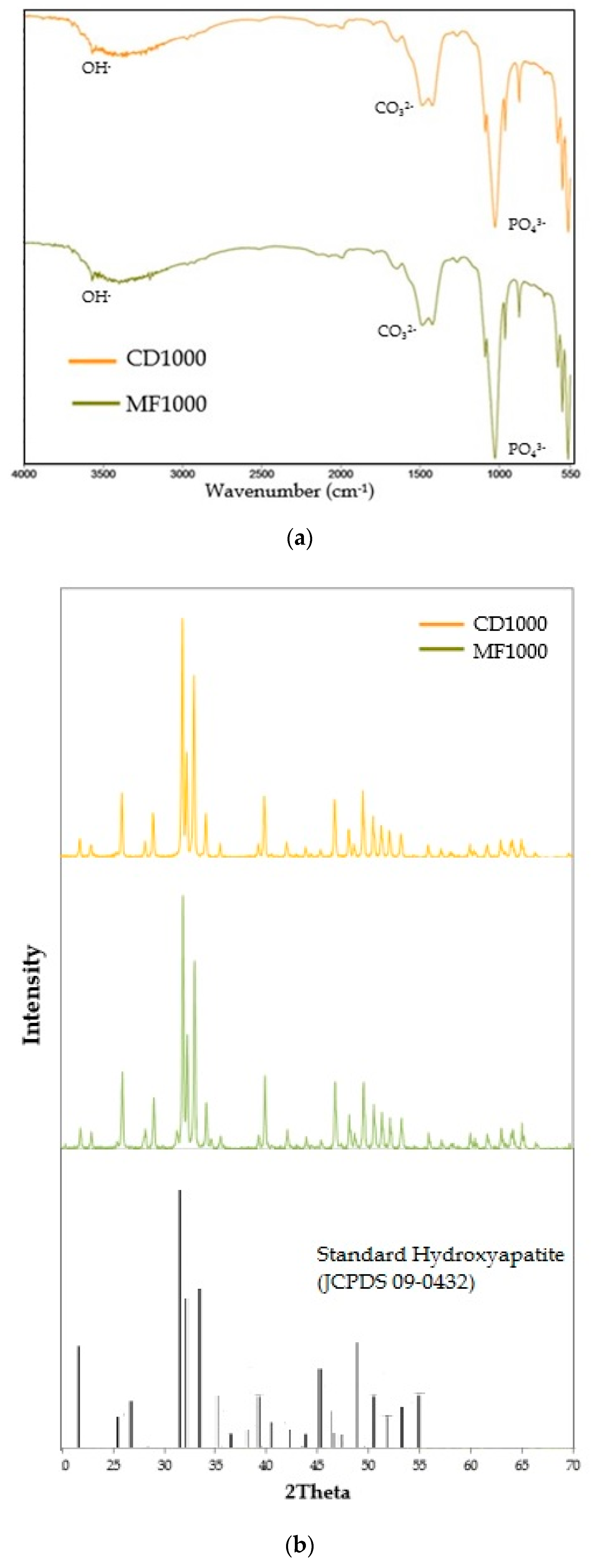
3.1. Characterization of Hydroxyapatite
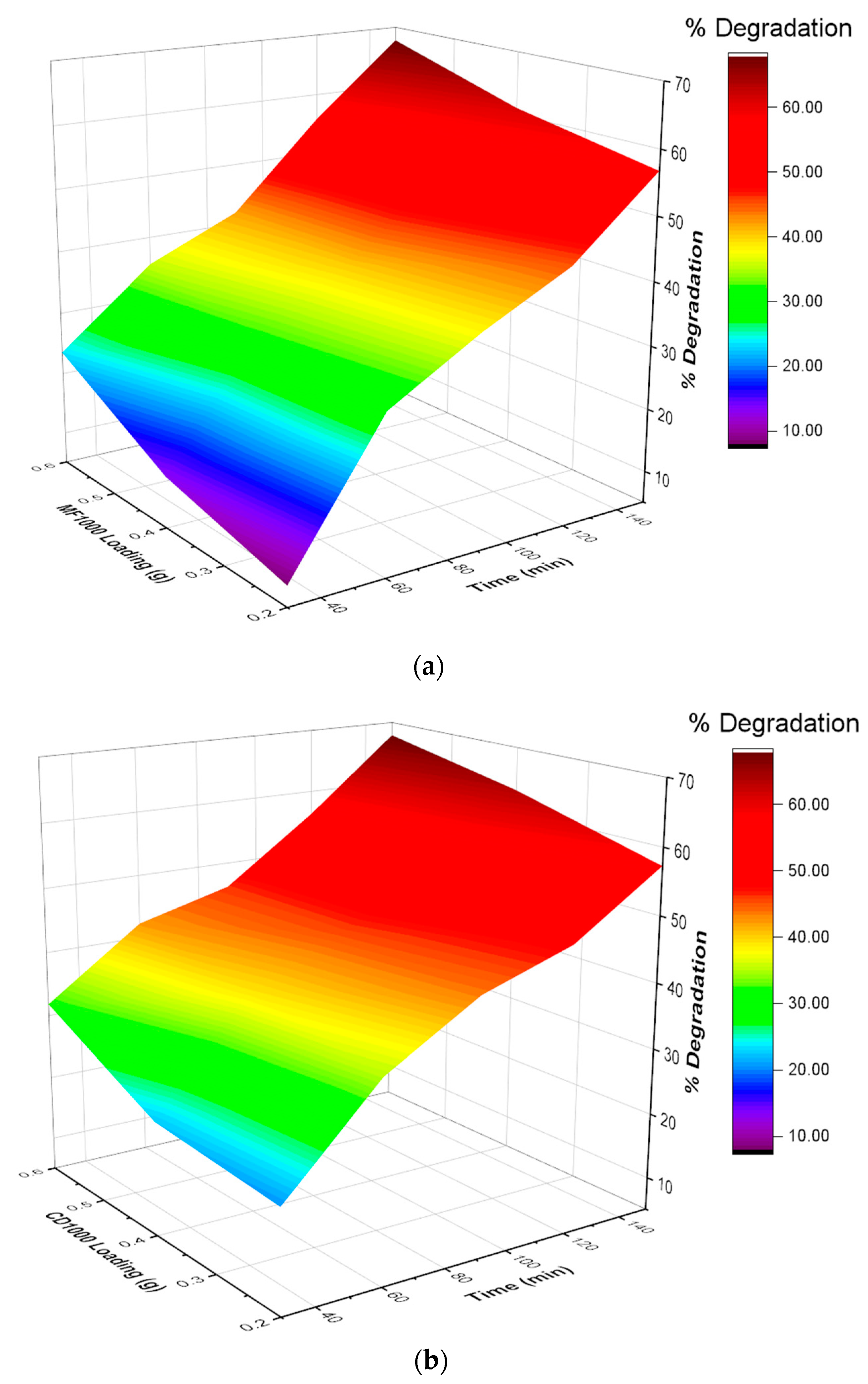
3.2. Photocatalytic Degradation of Malathion
3.3. Degradation Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.L.; Cosca, K.Z.; del Mundo, J. Trends of Pesticide Exposure and Related Cases in the Philippines. J. Rural Med. 2010, 5, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Varca, L.M. Pesticide residues in surface waters of Pagsanjan-Lumban catchment of Laguna de Bay, Philippines. Agric. Water Manag. 2012, 106, 35–41. [Google Scholar] [CrossRef]
- Elfman, L.; Tooke, N.E.; Patring, J.D. Detection of pesticides used in rice cultivation in streams on the island of Leyte in the Philippines. Agric. Water Manag. 2011, 101, 81–87. [Google Scholar] [CrossRef]
- Navarrete, I.A.; Tee, K.A.M.; Unson, J.R.S.; Hallare, A.V. Organochlorine pesticide residues in surface water and groundwater along Pampanga River, Philippines. Environ. Monit. Assess. 2018, 190, 289. [Google Scholar] [CrossRef] [PubMed]
- Bajet, C.; Kumar, A.; Calingacion, M.; Narvacan, T. Toxicological assessment of pesticides used in the Pagsanjan-Lumban catchment to selected non-target aquatic organisms in Laguna Lake, Philippines. Agric. Water Manag. 2012, 106, 42–49. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Trellu, C.; Vargas, H.O.; Mousset, E.; Oturan, N.; Oturan, M.A. Electrochemical technologies for the treatment of pesticides. Curr. Opin. Electrochem. 2021, 26, 100677. [Google Scholar] [CrossRef]
- Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Davis, L.C.; Erickson, L.E.; Al-Khatib, K.; Kulakow, P.; Barnes, P.L.; Hutchinson, S.; Nurzhanova, A.A. Potential for Plant-Based Remediation of Pesticide-Contaminated Soil and Water using Nontarget Plants such as Trees, Shrubs, and Grasses. Crit. Rev. Plant Sci. 2004, 23, 91–101. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Schnorr, C.; Oviedo, L.R.; Knani, S.; Silva, L.F.O.; Silva, W.L.; Dotto, G.L.; Rhoden, C.R.B. Adsorption and Photocatalytic Degradation of Pesticides into Nanocomposites: A Review. Molecules 2022, 27, 6261. [Google Scholar] [CrossRef] [PubMed]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Lelis, M.; Enesca, A.; Karazhanov, S. Photocatalytic activity of defective black-titanium oxide photocatalysts towards pesticide degradation under UV/VIS irradiation. Surf. Interfaces 2022, 32, 102123. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Rocha, R.L.P.; Honorio, L.M.C.; Bezerra, R.D.d.S.; Trigueiro, P.; Duarte, T.M.; Fonseca, M.G.; Silva-Filho, E.C.; Osajima, J.A. Light-Activated Hydroxyapatite Photocatalysts: New Environmentally-Friendly Materials to Mitigate Pollutants. Minerals 2022, 12, 525. [Google Scholar] [CrossRef]
- Mariappan, A.; Pandi, P.; Rajeswarapalanichamy, R.; Neyvasagam, K.; Sureshkumar, S.; Gatasheh, M.K.; Hatamleh, A.A. Bandgap and visible-light-induced photocatalytic performance and dye degradation of silver doped HAp/TiO2 nanocomposite by sol-gel method and its antimicrobial activity. Environ. Res. 2022, 211, 113079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Lu, L.; Liu, M.; Yuan, Z.; Yang, L.; Liu, C.; Huang, S.; Rao, Y. Highly efficient decontamination of tetracycline and pathogen by a natural product-derived Emodin/HAp photocatalyst. Chemosphere 2022, 305, 135401. [Google Scholar] [CrossRef] [PubMed]
- Rubi, R.V.; Roque, E.; Rosa, F.D.; Estoque, R.M.; Olvido, G.; Perey, P.J.; Teresa, J.S.; Tesalona, M.A. Photocatalytic degradation of Atrazine herbicide using nano-Hydroxyapatite from Cow Bone synthesized via Simulated Body Fluid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012013. [Google Scholar] [CrossRef]
| DF | Sum of Squares | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|
| Model | 21 | 6946.0871 | 330.7661 | 392.6802 | 7.4364 × 10−10 |
| Time ** | 4 | 6233.6492 | 1558.4123 | 1850.1223 | 6.7926 × 10−12 |
| Loading ** | 2 | 382.2993 | 191.1496 | 226.9298 | 9.0016 × 10−8 |
| HAp Type ** | 1 | 168.1328 | 168.1328 | 199.6045 | 6.1246 × 10−7 |
| Time x loading** | 8 | 76.3008 | 9.5376 | 11.3229 | 1.2400 × 10−3 |
| Time x HAp Type ** | 4 | 83.0029 | 20.7507 | 24.6349 | 1.4941 × 10−4 |
| Loading x HAp Type | 2 | 2.7022 | 1.3511 | 1.6040 | 2.5956 × 10−1 |
| MF1000 | CD1000 | |||||
|---|---|---|---|---|---|---|
| 0.2 g | 0.4 g | 0.6 g | 0.2 g | 0.4 g | 0.6 g | |
| First-Order Kinetics | ||||||
| k1 (×10−3), min−1 | 5.2503 | 6.0738 | 7.0289 | 6.0689 | 6.9224 | 7.7919 |
| Std. Error (×10−3) | 0.3120 | 0.2110 | 0.3727 | 0.3415 | 0.3619 | 0.7419 |
| Reduced Χ2 | 0.1404 | 0.0536 | 0.1359 | 0.1404 | 0.1311 | 0.4584 |
| SSE | 0.5615 | 0.2143 | 0.5436 | 0.5614 | 0.5245 | 1.8336 |
| R2 | 0.9577 | 0.9845 | 0.9547 | 0.9279 | 0.9448 | 0.7586 |
| Second-Order Kinetics | ||||||
| k2 (×10−3), L min−1 mg−1 | 0.6920 | 0.8325 | 1.0174 | 0.8520 | 1.0068 | 1.1946 |
| Std. Error (×10−3) | 0.0757 | 0.0790 | 0.0963 | 0.0248 | 0.0439 | 0.1044 |
| Reduced Χ2 | 0.2899 | 0.2316 | 0.2392 | 0.0220 | 0.0507 | 0.2057 |
| SSE | 1.1598 | 0.9262 | 0.9568 | 0.0880 | 0.2030 | 0.8229 |
| R2 | 0.9127 | 0.9331 | 0.9202 | 0.9887 | 0.9786 | 0.8917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Credo, A.S.; Pascual, M.G.; Villagracia, M.J.C.; Villaruz, A.D.; Roque, E.C.; Lopez, E.C.R.; Rubi, R.V.C. Photocatalytic Degradation of Malathion Using Hydroxyapatite Derived from Chanos chanos and Pangasius dory Bones. Eng. Proc. 2023, 37, 7. https://doi.org/10.3390/ECP2023-14618
Credo AS, Pascual MG, Villagracia MJC, Villaruz AD, Roque EC, Lopez ECR, Rubi RVC. Photocatalytic Degradation of Malathion Using Hydroxyapatite Derived from Chanos chanos and Pangasius dory Bones. Engineering Proceedings. 2023; 37(1):7. https://doi.org/10.3390/ECP2023-14618
Chicago/Turabian StyleCredo, Allen S., Mckenneth G. Pascual, Mark Jerome C. Villagracia, Alden D. Villaruz, Erison C. Roque, Edgar Clyde R. Lopez, and Rugi Vicente C. Rubi. 2023. "Photocatalytic Degradation of Malathion Using Hydroxyapatite Derived from Chanos chanos and Pangasius dory Bones" Engineering Proceedings 37, no. 1: 7. https://doi.org/10.3390/ECP2023-14618
APA StyleCredo, A. S., Pascual, M. G., Villagracia, M. J. C., Villaruz, A. D., Roque, E. C., Lopez, E. C. R., & Rubi, R. V. C. (2023). Photocatalytic Degradation of Malathion Using Hydroxyapatite Derived from Chanos chanos and Pangasius dory Bones. Engineering Proceedings, 37(1), 7. https://doi.org/10.3390/ECP2023-14618






