Automated and Enhanced Leucocyte Detection and Classification for Leukemia Detection Using Multi-Class SVM Classifier †
Abstract
:1. Introduction
2. Literature Review
3. Proposed Framework
3.1. Dataset Description
3.2. Image Pre-Processing
3.3. Feature Extraction
3.4. Feature Dimension Reduction
3.5. Classification
3.6. Multi-Class Classification
4. Result and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sajjad, M.; Khan, S.; Shoaib, M.; Ali, H.; Jan, Z.; Muhammad, K.; Mehmood, I. Computer Aided System for Leukocytes Classification and Segmentation in Blood Smear Images. In Proceedings of the 2016 International Conference on Frontiers of Information Technology (FIT), Islamabad, Pakistan, 19–21 December 2016; pp. 99–104. [Google Scholar] [CrossRef]
- Deng, L. An accurate segmentation method for white blood cell images. In Proceedings of the IEEE International Symposium on Biomedical Imaging, Washington, DC, USA, 7–10 July 2002; pp. 245–248. [Google Scholar]
- Yang, L.; Meer, P.; Foran, D. Unsupervised Segmentation Based on Robust Estimation and Color Active Contour Models. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Chongxun, Z.; Chen, P.; Li, L. White blood cell image segmentation using on-line trained neural network. In Proceedings of the 27th International Conference on Engineering in Medicine and Biology Society, Shanghai, China, 17–18 January 2006; pp. 6476–6479. [Google Scholar]
- Sholeh, F.I. White blood cell segmentation for fresh blood smear images. In Proceedings of the 2013 International Conference on Advanced Computer Science and Information Systems (ICACSIS), Sanur Bali, Indonesia, 28–29 September 2013; pp. 425–429. [Google Scholar] [CrossRef]
- Nazlibilek, S.; Karacor, D.; Ercan, T.; Sazli, M.H.; Kalender, O.; Ege, Y. Automatic segmentation, counting, size determination and classification of white blood cells. Measurement 2014, 55, 58–65. [Google Scholar] [CrossRef]
- Won, C.; Nam, J.Y.; Choe, Y. Segmenting cell images: A deterministic relaxation approach. In Computer Vision and Mathematical Methods in Medical and Biomedical Image Analysis; Springer: Berlin/Heidelberg, Germany, 2004; pp. 281–291. [Google Scholar]
- Ravikumar, S. Image segmentation and classification of white blood cells with the extreme learning machine and the fast rel-evance vector machine. Nanomed. Biotechnol. 2016, 44, 985–989. [Google Scholar]
- Dorini, L.B.; Minetto, R.; Leite, N.J. White blood cell segmentation using morphological operators and scale-space analysis. In Proceedings of the IEEE XX Brazilian Symposium on Computer Graphics and Image Processing, Minas, Gerais, Brazil, 2007; pp. 294–304. [Google Scholar]
- Alreza, Z.K.K.; Karimian, A. Design a new algorithm to count white blood cells for classification Leukemic Blood Image using machine vision system. In Proceedings of the 2016 6th International Conference on Computer and Knowledge Engineering (ICCKE), Mashhad, Iran, 20 October 2016. [Google Scholar]
- Morea, M.P.; Sugandhib, R. A Review on Systematic Investigation of Leucocytes Identification and Classification Techniques for Microscopic Blood Smear. Comput. Biol. Med. 2020, 116, 103530. [Google Scholar]
- Zamani, F.; Safabakhsh, R. An unsupervised GVF snake approach for white blood cell segmentation based on nucleus. In Proceedings of the 2006 8th international Conference on Signal Processing, Guilin, China, 16–20 November 2006; Volume 2. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Bodzas, A.; Kodytek, P.; Zidek, J. Automated Detection of Acute Lymphoblastic Leukemia From Microscopic Images Based on Human Visual Perception. Front. Bioeng. Biotechnol. 2020, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Dorini, L.B.; Minetto, R.; Leite, N.J. Semiautomatic White Blood Cell Segmentation Based on Multiscale Analysis. IEEE J. Biomed. Health Inf. 2012, 17, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bikhet, S.; Darwish, A.; Tolba, H.; Shaheen, S. Segmentation and classification of white blood cells. Contrast Media Mol. Imaging 2002, 4, 2259–2261. [Google Scholar] [CrossRef]
- Janani, S.D.; Selvi, R.M.; Mlndhu, G. Notice of Violation of IEEE Publication Principles: Blood Cell Detection and Counting Using Convolutional Sparse Dictionary Learning. In Proceedings of the 2018 International Conference on Current Trends towards Converging Technologies (ICCTCT), Coimbatore, India, 1–3 March 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Manik, S.; Saini, L.M.; Vadera, N. Counting and classification of white blood cell using Artificial Neural Network (ANN). In Proceedings of the 2016 IEEE 1st International Conference on Power Electronics, Intelligent Control and Energy Systems (ICPEICES), Delhi, India, 4–6 July 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Labati, R.D.; Piuri, V.; Scotti, F. All-IDB: The acute lymphoblastic leukemia image database for image processing. In Proceedings of the 011 18th IEEE International Conference on Image Processing, Sarajevo, Bosnia and Herzegovina, 16–18 June 2011; pp. 2045–2048. [Google Scholar] [CrossRef]
- Gautam, A.; Bhadauria, H. Classification of white blood cells based on morphological features. In Proceedings of the 2014 International Conference on Advances in Computing, Communications and Informatics (ICACCI), Delhi, India, 24–27 September 2014; pp. 2363–2368. [Google Scholar] [CrossRef]
- Yampri, P.; Pintavirooj, C.; Daochai, S.; Teartulakarn, S. White Blood Cell Classification based on the Combination of Eigen Cell and Parametric Feature Detection. In Proceedings of the IEEE Conference Industrial Electronics and Applications, Singapore, 24–26 May 2006; pp. 1–4. [Google Scholar] [CrossRef]
- Prinyakupt, J.; Pluempitiwiriyawej, C. Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. Biomed. Eng. Online 2015, 14, 63. [Google Scholar] [CrossRef] [PubMed]



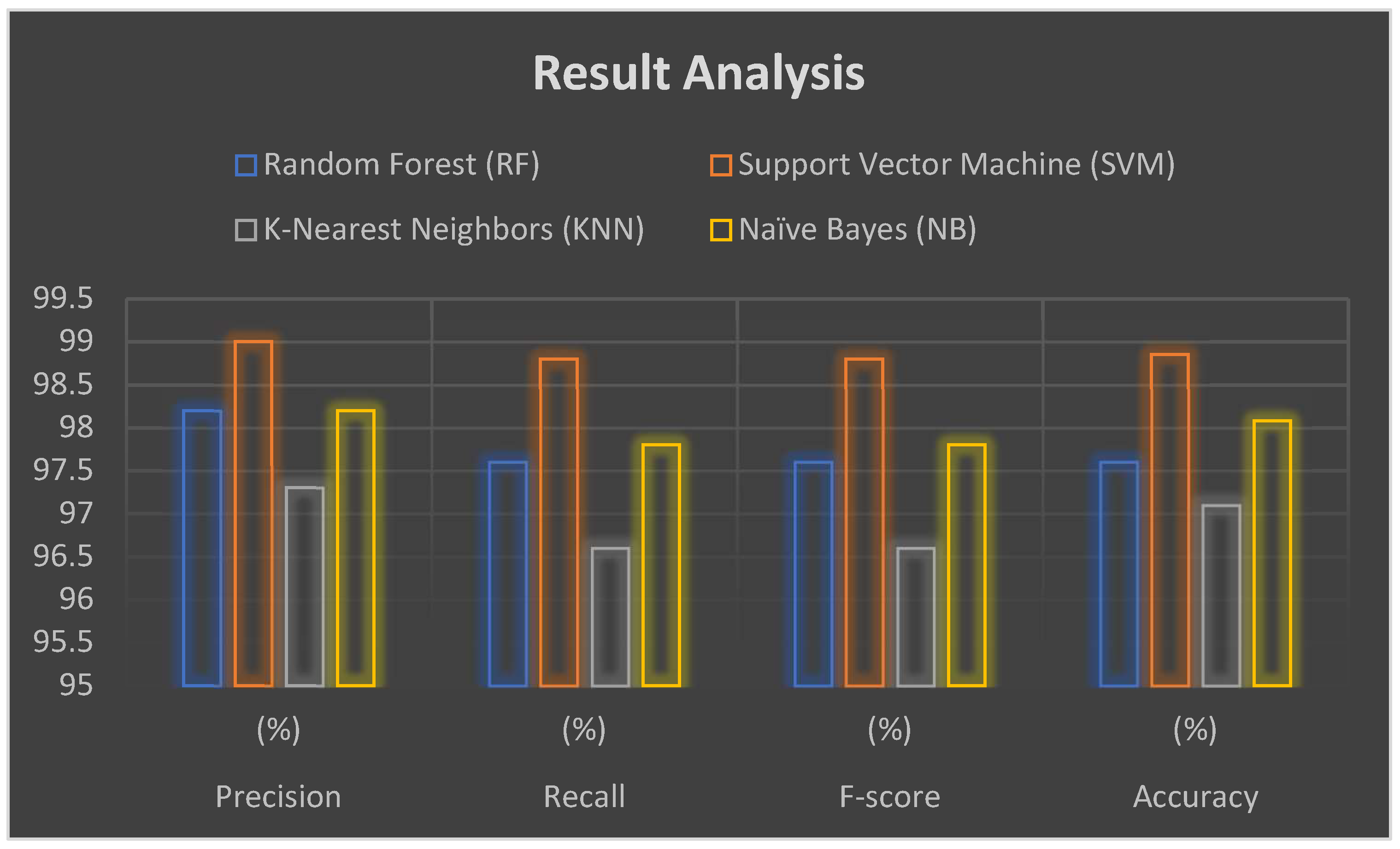
| Classifier | Precision (%) | Recall (%) | F-Score (%) | Accuracy (%) |
|---|---|---|---|---|
| Random Forest | 98.2 | 97.60 | 97.60 | 97.60 |
| Support Vector Machine | 99.00 | 98.80 | 98.80 | 98.85 |
| K-Nearest Neighbors | 97.30 | 96.60 | 96.60 | 97.10 |
| Naïve Bayes | 98.20 | 97.80 | 97.80 | 98.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, P.; Sugandhi, R. Automated and Enhanced Leucocyte Detection and Classification for Leukemia Detection Using Multi-Class SVM Classifier. Eng. Proc. 2023, 37, 36. https://doi.org/10.3390/ECP2023-14710
More P, Sugandhi R. Automated and Enhanced Leucocyte Detection and Classification for Leukemia Detection Using Multi-Class SVM Classifier. Engineering Proceedings. 2023; 37(1):36. https://doi.org/10.3390/ECP2023-14710
Chicago/Turabian StyleMore, Pranav, and Rekha Sugandhi. 2023. "Automated and Enhanced Leucocyte Detection and Classification for Leukemia Detection Using Multi-Class SVM Classifier" Engineering Proceedings 37, no. 1: 36. https://doi.org/10.3390/ECP2023-14710
APA StyleMore, P., & Sugandhi, R. (2023). Automated and Enhanced Leucocyte Detection and Classification for Leukemia Detection Using Multi-Class SVM Classifier. Engineering Proceedings, 37(1), 36. https://doi.org/10.3390/ECP2023-14710





