Beeswax and Castor Oil to Improve the Moisture Barrier and Tensile Properties of Pectin-Based Edible Films for Food Packaging Applications †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Development
2.2. Characterization
2.2.1. Moisture Content and Water Solubility
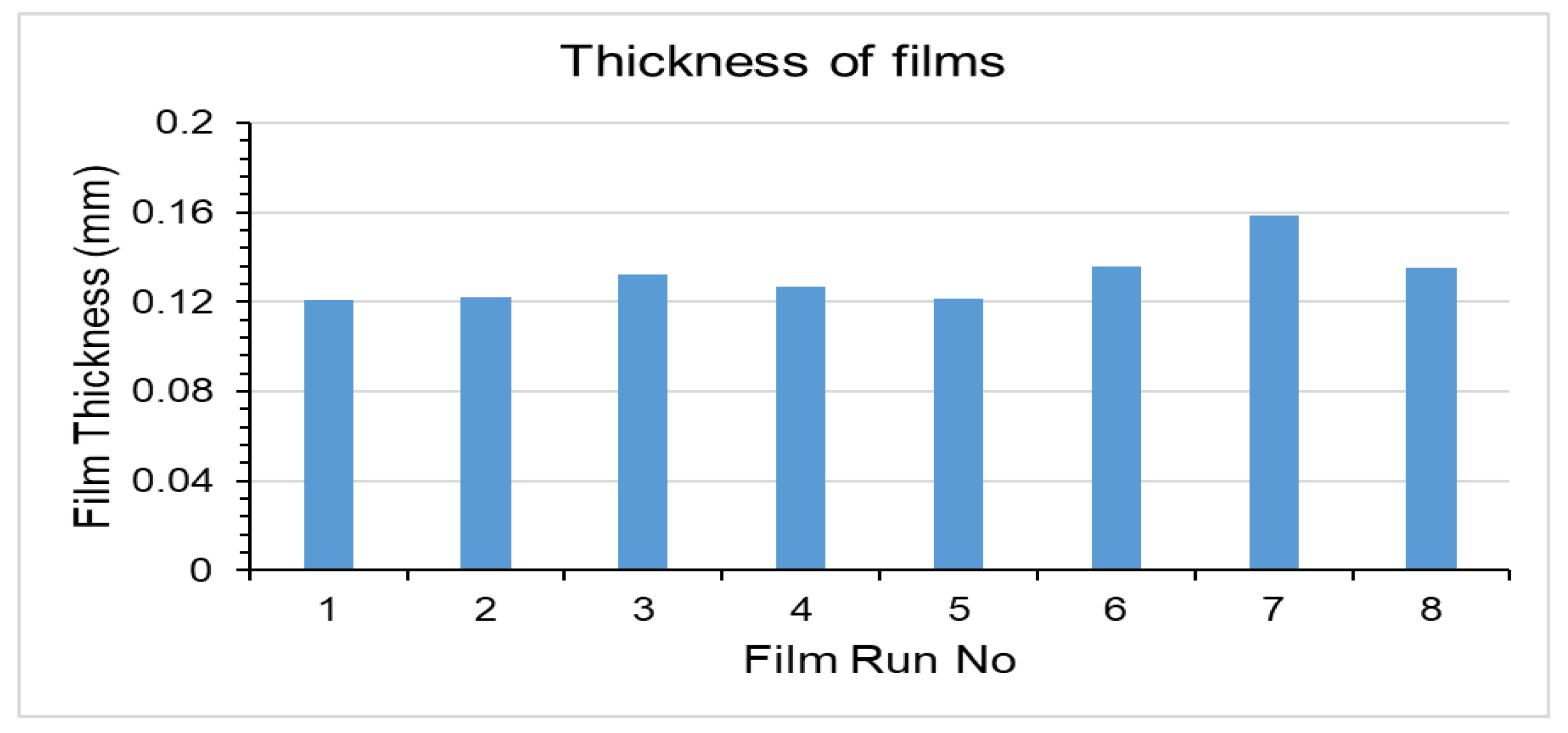
2.2.2. Thickness
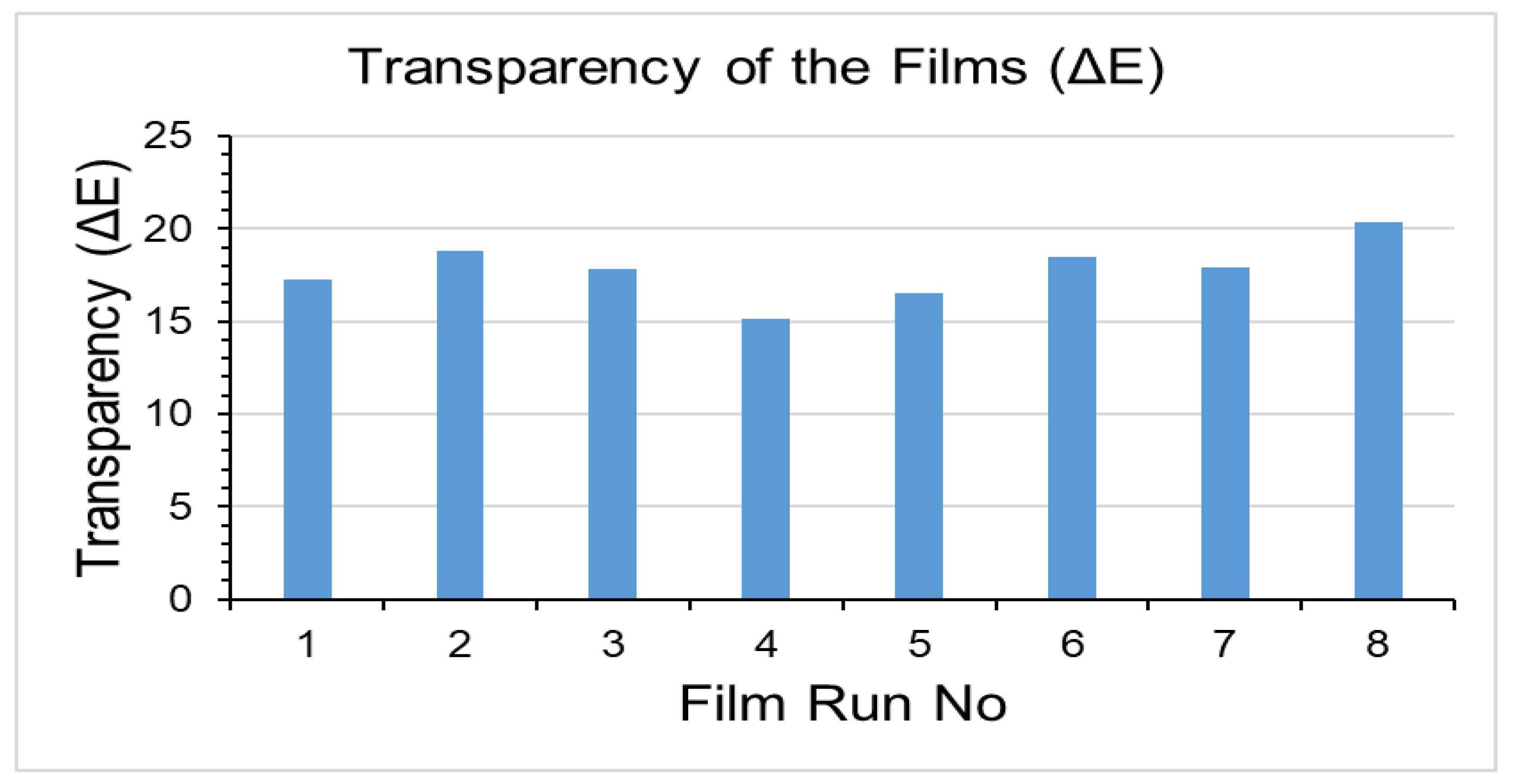
2.2.3. Optical Property or Transparency
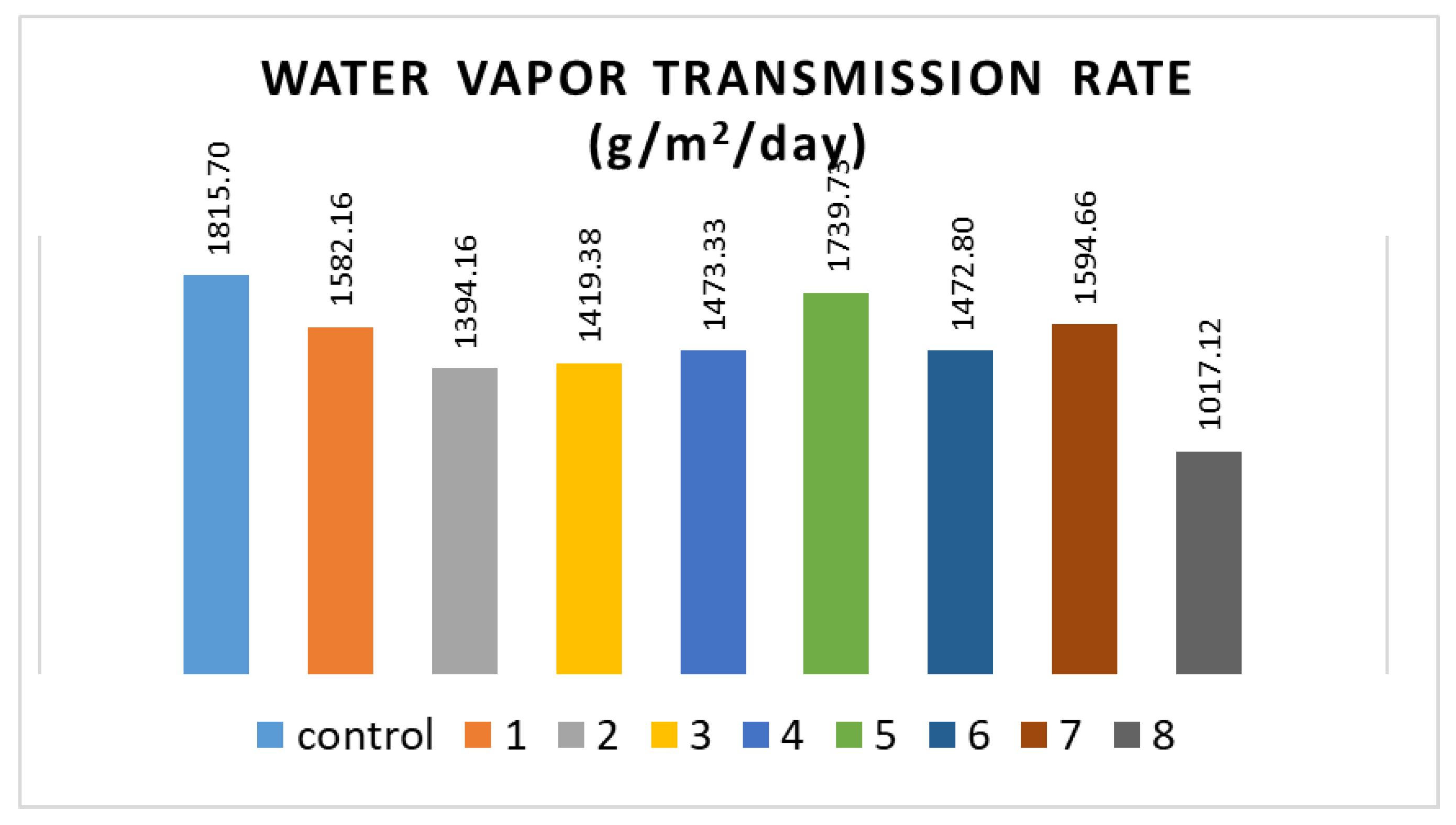
2.2.4. WVTR
2.2.5. Water Contact Angle and Mechanical Properties
2.2.6. FTIR
3. Results and Discussion
3.1. Water Solubility
3.2. Thickness
3.3. Transparency
3.4. WVTR
3.5. Water Contact Angle and Mechanical Properties
3.6. FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehraj, S.; Sistla, Y.S. Optimization of process conditions for the development of pectin and glycerol based edible films: Statistical design of experiments. Electron. J. Biotechnol. 2022, 55, 27–39. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Cortes-Rodríguez, M.; Villegas-Yepez, C.; Gonzalez, J.H.G.; Rodríguez, P.E.; Ortega-Toro, R. Development and evaluation of edible films based on cassava starch, whey protein, and bees wax. Heliyon 2020, 6, E04884. [Google Scholar] [CrossRef] [PubMed]
- Food Science and Quality Management ISSN 2224-6088 (Paper) ISSN 2225-0557 (Online) Volume 3. 2012. Available online: www.iiste.org (accessed on 16 May 2023).
- Mehraj, S.; Pandey, G.; Garg, M.; Santra, B.; Grewal, H.S.; Kanjilal, A.; Sistla, Y.S. Castor Oil and Cocoa Butter to Improve the Moisture Barrier and Tensile Properties of Pectin Films. J. Polym. Environ. 2023, 31, 312–326. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Flôres, S.H. Biodegradable Films Based on Gelatin and Papaya Peel Microparticles with Antioxidant Properties. Food Bioprocess Technol. 2018, 11, 536–550. [Google Scholar] [CrossRef]

| Run No | Castor Oil (% w/w of Pectin) | Beeswax (% w/w of Pectin) | Clove Oil (% w/w of Pectin) |
|---|---|---|---|
| 1 | 5 (low) | 5 (low) | 2 (low) |
| 2 | 15 (high) | 5 | 2 |
| 3 | 5 | 10 (high) | 2 |
| 4 | 15 | 10 | 2 |
| 5 | 5 | 5 | 4 (high) |
| 6 | 15 | 5 | 4 |
| 7 | 5 | 10 | 4 |
| 8 | 15 | 10 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharhshini, A.; Sistla, Y.S. Beeswax and Castor Oil to Improve the Moisture Barrier and Tensile Properties of Pectin-Based Edible Films for Food Packaging Applications. Eng. Proc. 2023, 37, 33. https://doi.org/10.3390/ECP2023-14670
Dharhshini A, Sistla YS. Beeswax and Castor Oil to Improve the Moisture Barrier and Tensile Properties of Pectin-Based Edible Films for Food Packaging Applications. Engineering Proceedings. 2023; 37(1):33. https://doi.org/10.3390/ECP2023-14670
Chicago/Turabian StyleDharhshini, A, and Yamini Sudha Sistla. 2023. "Beeswax and Castor Oil to Improve the Moisture Barrier and Tensile Properties of Pectin-Based Edible Films for Food Packaging Applications" Engineering Proceedings 37, no. 1: 33. https://doi.org/10.3390/ECP2023-14670
APA StyleDharhshini, A., & Sistla, Y. S. (2023). Beeswax and Castor Oil to Improve the Moisture Barrier and Tensile Properties of Pectin-Based Edible Films for Food Packaging Applications. Engineering Proceedings, 37(1), 33. https://doi.org/10.3390/ECP2023-14670






