Supercritical Fluid CO2 Extraction Technology to Produce an Innovative Healthy Product from Almond Wastes †
Abstract
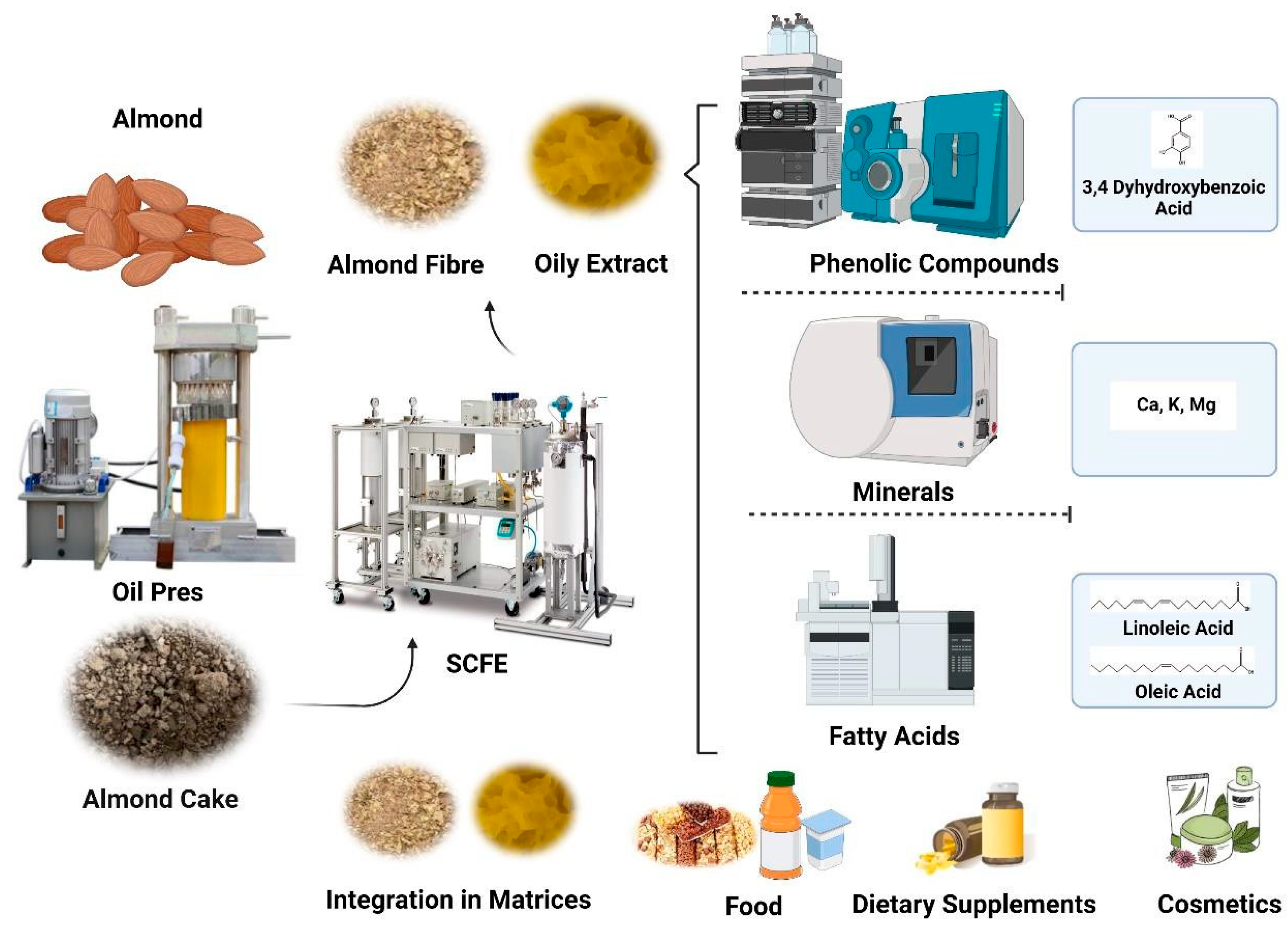
:1. Introduction
2. Material and Method Section
2.1. By-Products Sample Preparation and Supercritical Fluid Extraction (SFE-CO2)
2.2. Analysis of Phenols by LC-MS/MS
2.3. Ash Content and Analysis of Minerals by ICP-OES
2.4. Fatty Acid Analysis
3. Results and Discussion
3.1. Analysis of Phenolic Family Compounds
3.2. Analysis of Ash Content and Minerals
3.3. Analysis of Fatty Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanahuja, A.B.; Pérez, S.E.M.; Teruel, N.G.; García, A.V.; Moya, M.S.P. Variability of chemical profile in almonds (Prunus dulcis) of different cultivars and origins. Foods 2021, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Food and Agriculture Organization of the United States, FAOSTAT Database. Available online: https://www.fao.org/statistics/en/ (accessed on 1 March 2023).
- Spanish Ministery of Agriculture Fish and Food. Superficies y Producciones Anuales de Cultivos. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 14 April 2023).
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond by-products: Valorization for sustainability and competitiveness of the industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A quick, green and simple ultrasound-assisted extraction for the valorization of antioxidant phenolic acids from moroccan almond cold-pressed oil residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Barba, F.J.; Moreno, A. Almond hull biomass: Preliminary characterization and development of two alternative valorization routes by applying innovative and sustainable technologies. Ind. Crops Prod. 2022, 179, 114697. [Google Scholar] [CrossRef]
- de Souza, T.S.P.; Dias, F.F.G.; Oliveira, J.P.S.; de Moura Bell, J.M.L.N.; Koblitz, M.G.B. Biological properties of almond proteins produced by aqueous and enzyme-assisted aqueous extraction processes from almond cake. Sci. Rep. 2020, 10, 10873. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Firouzi, M.; Dadmehr, M.; Javad-Mousavi, S.A.; Bagheriani, N.; Sadeghpour, O. Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: A narrative review. Phyther. Res. 2021, 35, 2997–3012. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- Ouzir, M.; Bernoussi, S.E.; Tabyaoui, M.; Taghzouti, K. Almond oil: A comprehensive review of chemical composition, extraction methods, preservation conditions, potential health benefits, and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3344–3387. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O.; Abbas, K. Analysis of volatile flavour compounds and acrylamide in roasted Malaysian tropical almond (Terminalia catappa) nuts using supercritical fluid extraction. Food Chem. Toxicol. 2010, 48, 2212–2216. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Distribution and exudation of allelochemicals in wheat Triticum aestivum. J. Chem. Ecol. 2000, 26, 2141–2154. [Google Scholar] [CrossRef]
- Biocombustibles, T.N. Solid Biofuels. Method for Determination of Ash Content UNE-EN 14775. Norma Española 2010. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0040183 (accessed on 14 April 2023).
- Millos, J.; Costas-rodríguez, M.; Lavilla, I.; Bendicho, C. Multiple small volume microwave-assisted digestions using conventional equipment for multielemental analysis of human breast biopsies by inductively coupled plasma optical emission spectrometry. Talanta 2009, 77, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; Garcia-Risco, M. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood Waste from Fruit Trees: Biomolecules and Their Applications in Agri-Food Industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.; Natić, M.M. Fatty acid and phenolic profiles of almond grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kahlaoui, M.; Borotto, S.; Vecchia, D.; Giovine, F.; Ben, H.; Kbaier, H.; Bouzouita, N.; Pereira, L.B. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
| SCFE of Almond Cake | ||||||
|---|---|---|---|---|---|---|
| COMPOUND | ABREV. | Almond Cake | Fibre Ingredient | Fibre Ingredient | Oily Extract | Oily Extract |
| 20 MPa | 24 MPa | 20 MPa | 24 MPa | |||
| Phenolic acids | ||||||
| 3,4-dihydroxybenzoic acid | DA | 54.40 | 57.40 | 35.00 | 1.65 | 4.18 |
| Vanillic acid | VA | 7.06 | 7.41 | 5.06 | 6.88 | 6.15 |
| Syringic acid | SA | 1.99 | 2.01 | 1.60 | 0.35 | 0.10 |
| Protocatechuic acid | PTA | 0.64 | 0.62 | 0.48 | nd | nd |
| Salicylic acid | SAA | 0.22 | 0.24 | 0.17 | nd | nd |
| Ferulic acid | FA | 0.25 | 0.25 | 0.21 | 0.34 | 0.44 |
| p-coumaric acid | P-CA | 0.13 | 0.13 | 0.09 | 0.30 | 0.63 |
| Phthalic acid | PA | 0.25 | 0.24 | 0.16 | 0.04 | 0.04 |
| Flavonoids | ||||||
| Rutin | RU | 0.54 | 0.554 | 0.422 | nd | nd |
| Quercetin | QE | 0.12 | 0.12 | 0.13 | 0.16 | 0.05 |
| Vanillin | VN | 13.50 | 16.49 | 9.23 | 0.55 | 0.39 |
| Luteolin | LU | 0.01 | 0.01 | 0.02 | 0.04 | 0.01 |
| Aldehydes | ||||||
| Syringaldehyde | SY | 5.57 | 6.34 | 4.04 | 1.54 | 0.81 |
| SCFE of Almond Cake | |||||
|---|---|---|---|---|---|
| MINERAL | Almond Cake | Fibre Ingredient | Fibre Ingredient | Extract | Extract |
| 20 MPa | 24 MPa | 20 MPa | 24 MPa | ||
| Ash (%) | 2.63 | 2.74 | 2.72 | - | - |
| Macroelements (g/kg) | |||||
| Ca | 2.84 | 3.02 | 1.64 | 0.030 | 0.012 |
| K | 6.00 | 6.08 | 5.91 | 0.001 | 0.001 |
| Mg | 1.63 | 1.74 | 1.46 | 0.001 | 0.003 |
| P | 2.80 | 2.95 | 1.39 | 0.001 | 0.007 |
| Na | 0.34 | 0.32 | 0.46 | 1.11 | 0 |
| Microelements (mg/kg) | |||||
| Mn | 18.8 | 19.2 | 0.9 | 0.05 | 0.14 |
| Fe | 290.2 | 299.7 | 299 | 1.83 | 8.96 |
| Cu | 15.5 | 11.9 | 0.2 | 0.29 | 0.29 |
| Zn | 22.7 | 23.3 | 0.82 | 0.72 | |
| SCFE of Almond Cake | |||||
|---|---|---|---|---|---|
| FA | Almond Cake | Fibre Ingredient | Fibre Ingredient | Oily Extract | Oily Extract |
| 20 MPa | 24 MPa | 20 MPa | 24 MPa | ||
| Total SFAs | 42.53 | 15.17 | 10.22 | 676.91 | 633.45 |
| Total MUFAs | 27.93 | 10.09 | 6.91 | 453.65 | 437.22 |
| Total PUFAs | 10.83 | 3.66 | 2.48 | 153.00 | 119.77 |
| Total FA | 81.29 | 28.92 | 19.61 | 1283.56 | 1190.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, F.; Echave, J.; Prieto, M.A.; Simal-Gandara, J.; Otero, P. Supercritical Fluid CO2 Extraction Technology to Produce an Innovative Healthy Product from Almond Wastes. Eng. Proc. 2023, 37, 118. https://doi.org/10.3390/ECP2023-14712
Chamorro F, Echave J, Prieto MA, Simal-Gandara J, Otero P. Supercritical Fluid CO2 Extraction Technology to Produce an Innovative Healthy Product from Almond Wastes. Engineering Proceedings. 2023; 37(1):118. https://doi.org/10.3390/ECP2023-14712
Chicago/Turabian StyleChamorro, Franklin, Javier Echave, Miguel. A. Prieto, Jesus Simal-Gandara, and Paz Otero. 2023. "Supercritical Fluid CO2 Extraction Technology to Produce an Innovative Healthy Product from Almond Wastes" Engineering Proceedings 37, no. 1: 118. https://doi.org/10.3390/ECP2023-14712
APA StyleChamorro, F., Echave, J., Prieto, M. A., Simal-Gandara, J., & Otero, P. (2023). Supercritical Fluid CO2 Extraction Technology to Produce an Innovative Healthy Product from Almond Wastes. Engineering Proceedings, 37(1), 118. https://doi.org/10.3390/ECP2023-14712










