Photocatalytic Degradation and Defluorination of Per- and Poly-Fluoroalkyl Substances (PFASs) Using Biosynthesized TiO2 Nanoparticles under UV–Visible Light †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Nanoparticles
2.2. Characterization of TiO2 Nanoparticles
2.3. Photodegradation of PFOS
3. Results and Discussion
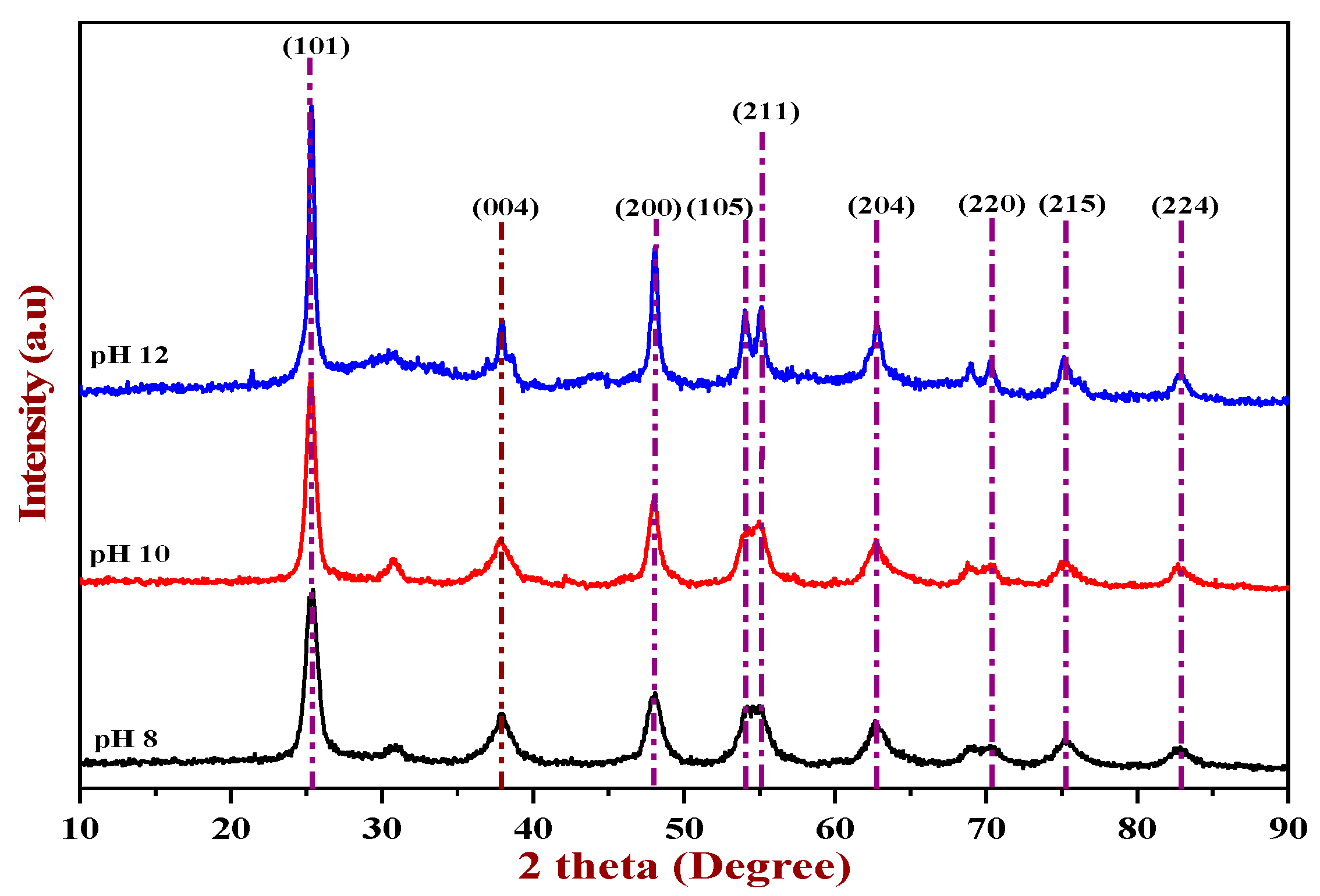
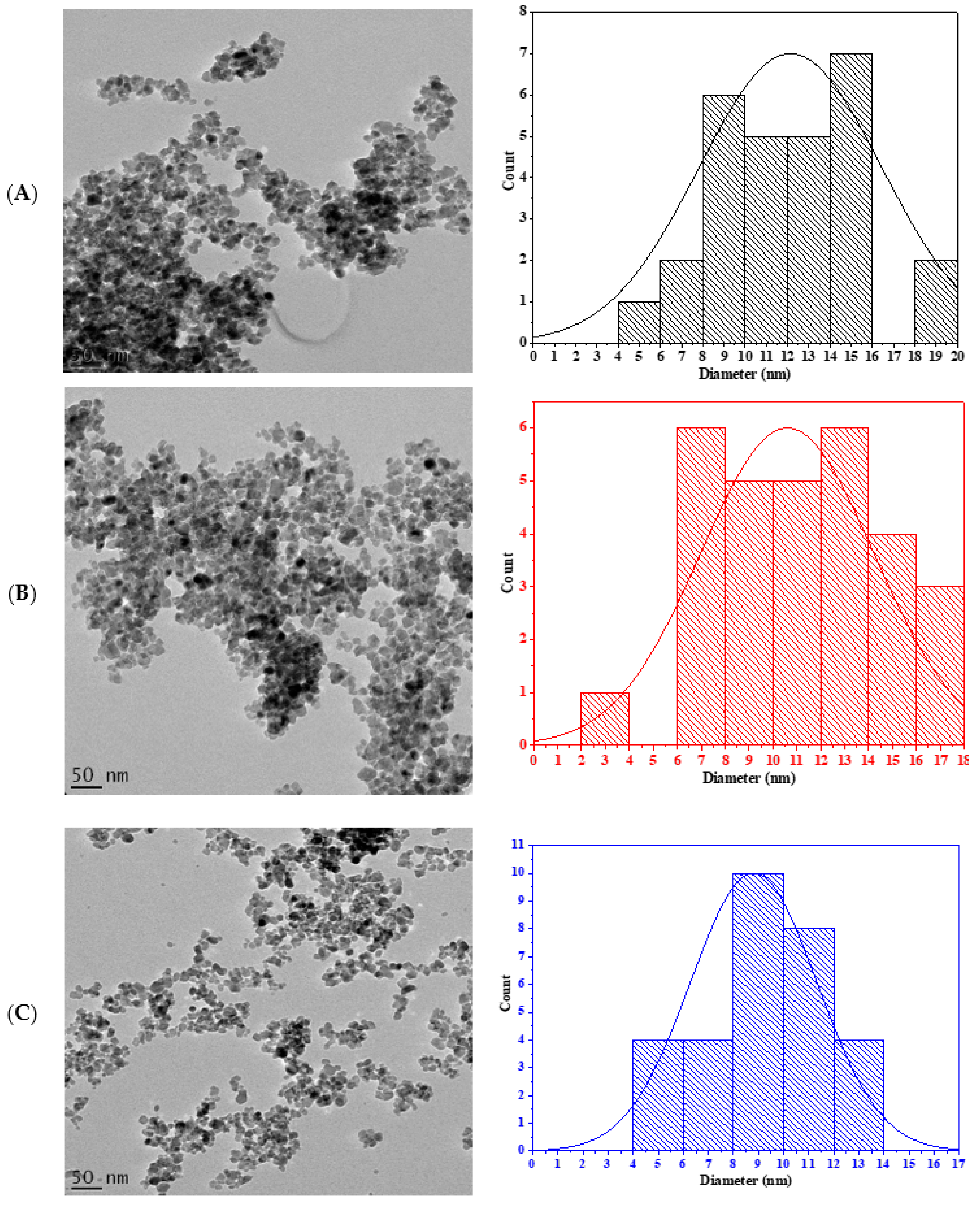
3.1. Characterization of TiO2 Nanoparticles
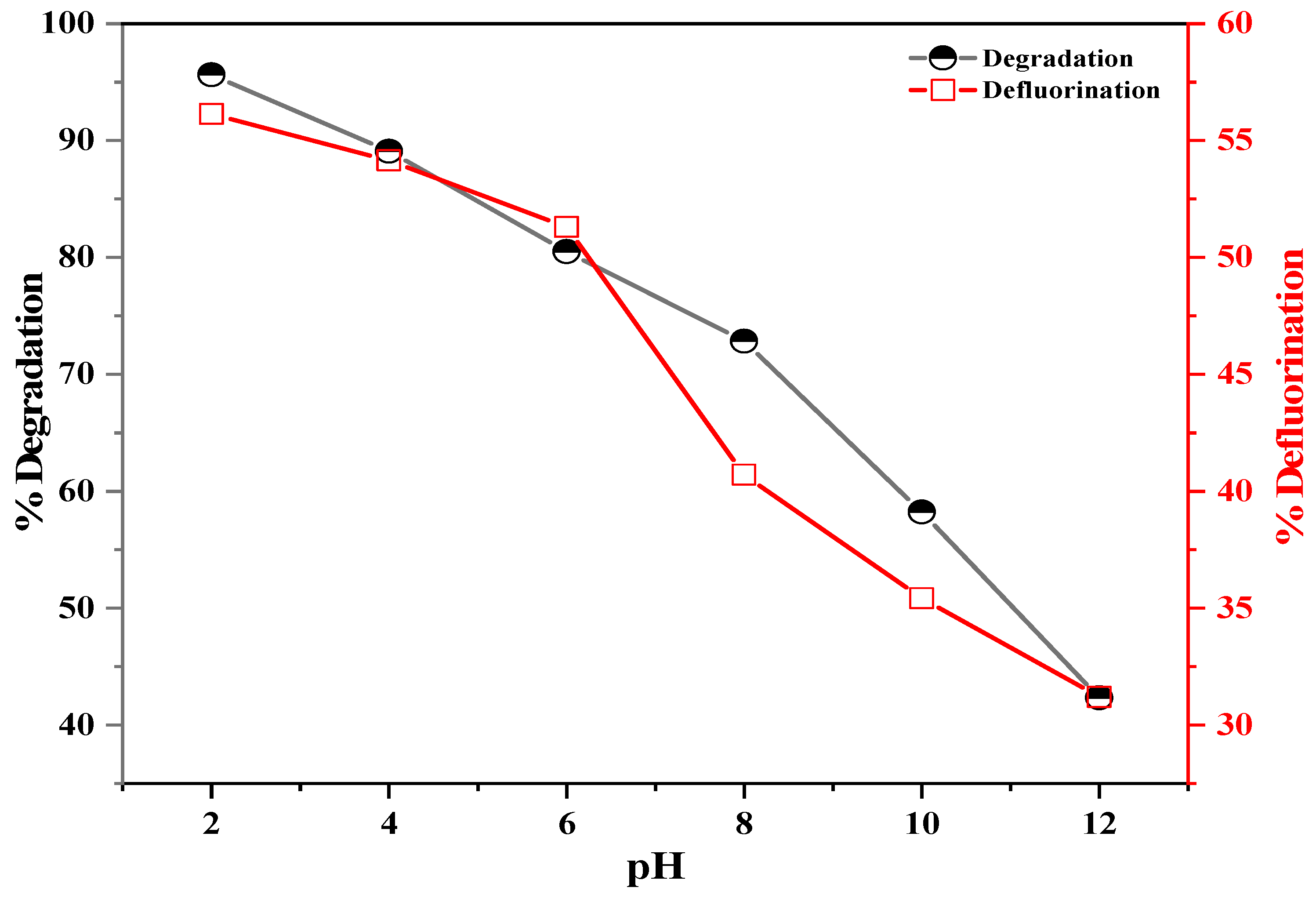
3.2. Photodegradation and Defluorination of PFOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olatunde, O.C.; Kuvarega, A.T.; Onwudiwe, D.C. Photo enhanced degradation of polyfluoroalkyl and perfluoroalkyl substances. Heliyon 2020, 6, e05614. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, J. Degradation of perfluorooctanoic acid by zero-valent iron nanoparticles under ultraviolet light. J. Nanoparticle Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Verma, S.; Mezgebe, B.; Sahle-Demessie, E.; Nadagouda, M.N. Photooxidative decomposition and defluorination of perfluorooctanoic acid (PFOA) using an innovative technology of UV–vis/ZnxCu1-xFe2O4/oxalic acid. Chemosphere 2021, 280, 130660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef]
- Zhang, W.; Efstathiadis, H.; Li, L.; Liang, Y. Environmental factors affecting degradation of perfluorooctanoic acid (PFOA) by In2O3 nanoparticles. J. Environ. Sci. 2020, 93, 48–56. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saheed, M.; Jimoh Oladejo, T.; Rabi, E.; Muhammed Binin, E.; Azeezah Taiwo, A.; Damola Taye, S.; Abdulmumuni, S.; Adekunle Jelili, O.; Hassana Ladio, A.; Saka Abdulkareem, A.; et al. Photocatalytic Degradation and Defluorination of Per- and Poly-Fluoroalkyl Substances (PFASs) Using Biosynthesized TiO2 Nanoparticles under UV–Visible Light. Eng. Proc. 2023, 37, 114. https://doi.org/10.3390/ECP2023-14630
Saheed M, Jimoh Oladejo T, Rabi E, Muhammed Binin E, Azeezah Taiwo A, Damola Taye S, Abdulmumuni S, Adekunle Jelili O, Hassana Ladio A, Saka Abdulkareem A, et al. Photocatalytic Degradation and Defluorination of Per- and Poly-Fluoroalkyl Substances (PFASs) Using Biosynthesized TiO2 Nanoparticles under UV–Visible Light. Engineering Proceedings. 2023; 37(1):114. https://doi.org/10.3390/ECP2023-14630
Chicago/Turabian StyleSaheed, Mustapha, Tijani Jimoh Oladejo, Elabor Rabi, Etsuyankpa Muhammed Binin, Amigun Azeezah Taiwo, Shuaib Damola Taye, Sumaila Abdulmumuni, Olaoye Adekunle Jelili, Abubakar Hassana Ladio, Abdulkareem Saka Abdulkareem, and et al. 2023. "Photocatalytic Degradation and Defluorination of Per- and Poly-Fluoroalkyl Substances (PFASs) Using Biosynthesized TiO2 Nanoparticles under UV–Visible Light" Engineering Proceedings 37, no. 1: 114. https://doi.org/10.3390/ECP2023-14630
APA StyleSaheed, M., Jimoh Oladejo, T., Rabi, E., Muhammed Binin, E., Azeezah Taiwo, A., Damola Taye, S., Abdulmumuni, S., Adekunle Jelili, O., Hassana Ladio, A., Saka Abdulkareem, A., & Muhammed Muhammed, N. (2023). Photocatalytic Degradation and Defluorination of Per- and Poly-Fluoroalkyl Substances (PFASs) Using Biosynthesized TiO2 Nanoparticles under UV–Visible Light. Engineering Proceedings, 37(1), 114. https://doi.org/10.3390/ECP2023-14630




