Abstract
Despite topical research, the study of flotation systems remains complex, multifaceted and water-intensive. Numerous physical and chemical factors are involved in the recovery of valuable minerals by flotation. While the chemistry of a system can be manipulated to improve the performance, the system is limited by the mineralogy of the incoming ore and the quality of the process water, which in most cases is not controlled. Recycling of onsite process water has become the norm for many operations; this recycling changes the water quality over time and may compromise the flotation process. This study seeks to understand the impact of ore feed grade on froth stability, entrainment, and flotation performance under varying water quality. The overarching aim is the development of a relationship through which the flotation performance may be predicted if the ore feed grade and water quality are known.
1. Introduction
1.1. Background
Mining operations cannot operate without continuous access to water, the majority of which is utilized by mineral processing and dust suppression. The need to find alternative sources of water that meet operational demands has forced many mining operations to recycle onsite process water. This recycling may be key to engineering design; however, it may compromise the flotation process and the efficiency of the flotation performance. A fundamental understanding of mineral processing is thus paramount to maximize the recovery of valuable minerals.
1.2. Mineralogy
The mineralogy of an ore determines its flotation performance, thereby making the mineralogy of the incoming ore paramount for achieving high process performance and concentrates. Different ores bear different minerals and thus behave differently in the presence of chemical reactants, making it difficult to isolate and quantify individual reagent interactions with minerals in the ore [1]. Although limited by surface liberation and particle size, a synthetic ore allows for the isolation of distinct mineral behavior and can thus be used for the purpose of investigating the impact of the ore feed grade on flotation performance under varying water quality.
1.3. Froth Flotation
Froth flotation is a physico-chemical separation process extensively used in mineral processing for separating minerals to concentrate them for economic smelting. The process utilizes the differences in surface properties of minerals to selectively separate valuable minerals from unwanted gangue [2].
Chemical reagents are added to a milled ore slurry to facilitate separation and enhance the difference in hydrophobicity between unwanted gangue and valuable minerals [3,4,5,6]. A typical reagent suite consists of collectors, depressants, frothers and sometimes activators [7,8].
A holistic understanding is essential to evaluate flotation reagent interactions in both the pulp phase and froth phase, as reagents may also interact with one another, in addition to their primary roles in the flotation process [9].
The manipulation of these reagents can improve performance; however, the system is limited by the mineralogy of the incoming ore as well as the quality of the process water, which in most cases is not controlled. The process utilizes a considerable amount of continuous water input, making water quality paramount for achieving high process performance and enrichment of valuables [10]. Recycling of onsite process water changes the water quality and may compromise the flotation process by affecting the rate of recovery, froth stability, mineral pulp and flotation performance. This adds complexity to the flotation process and its chemical reactants.
2. Froth Flotation Fundamentals
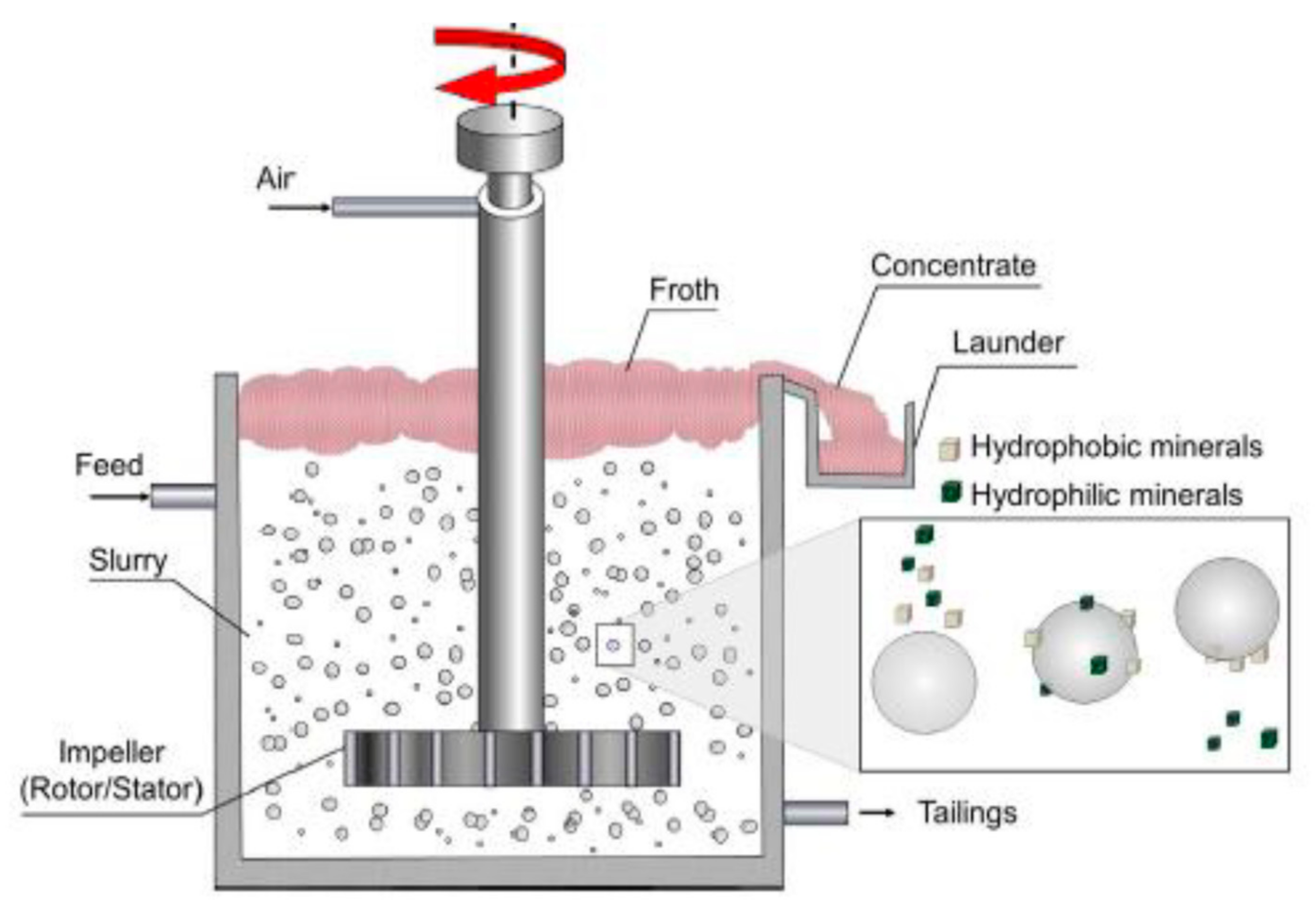
Flotation is modelled as a two-stage process; first, the pulp phase, whereby mineral recovery occurs, and second, the froth phase, whereby concentrated valuable minerals are separated from the bulk [11]. Figure 1 shows an illustrative diagram of the flotation process and its components. Suspended mineral particles from the flotation pulp report to the concentrate through three distinct mechanisms, namely, true flotation, entrainment and entrapment [12,13]. True flotation is a selective process responsible for the collection of valuable minerals, while entrainment and entrapment are non-selective processes responsible for the collection of both valuable and unwanted gangue minerals reporting to the froth phase [14,15]. True flotation is the dominant mechanism through which recovery of valuable minerals occurs.
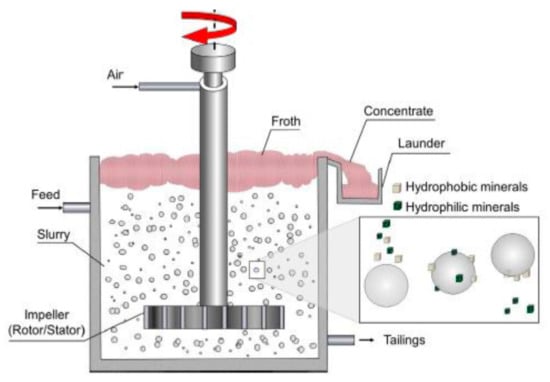
Figure 1.
An illustration a flotation cell with its components [2].
3. Froth Stability Fundamentals
During the flotation process, air bubbles are generated and pass through the flotation pulp into the froth phase, whereby they emerge surrounded by a thin liquid film. A plateau border with the liquid film develops when there are at least three bubbles clustering together [16]. Continuous clustering of bubbles results typically in the formation of polyhedral bubbles with films in between them; this is referred to as foam in the case of a two-phase system and known as froth in the case of a three-phase system containing solid particles [16].
The froth phase provides an environment for separating hydrophobic valuables from unwanted gangue and allows for the drainage of entrained material back into the flotation pulp [17]. For an optimum flotation performance, it is critical for flotation systems to exhibit an optimum froth stability [18].
Froth stability can be defined as the persistence of the froth or a measure of the froth’s lifetime [17,19]. When the froth is unstable, bubbles continuously break down before collection, owing to liquid drainage from entrained material back into the flotation pulp. When the froth is too stable, not enough liquid drainage occurs, and as a result, high water and gangue recoveries are achieved. The froth phase may be characterised by the froth bulk and froth surface; froth surface stability is related to the bursting of bubbles into the atmosphere, while froth bulk stability is reflected by the size of bubbles on the froth surface.
4. Factors Affecting Froth Stability
Froth stability can be affected by several factors: mainly, particle properties, operational conditions and chemistry effects such as collectors, frothers and depressants [19]. Particle properties such as particle hydrophobicity, shape and size are of great importance to bubble particle attachment and significantly affect froth stability; thus have a substantial effect on the overall flotation performance, an extent which can be greater than the beneficial effects in the pulp phase [20]. These particle property effects have been shown to significantly affect froth stability with increasing distance from the pulp/froth interface, where the bubble films are thinner to allow for bridging to occur. Particle properties are said to have a greater influence on froth stability in comparison to operational factors (aeration rate, froth height and gas dispersion) and chemistry effects [20].
5. Chemistry Effects on the Stability of the Froth
Collectors impart particle hydrophobicity of minerals and can thus have a significant effect on froth stability, owing to the difference in the degree of hydrophobicity imparted onto the mineral particles [21]. Depressants suppress the floatability of naturally floatable hydrophobic gangue minerals and thus improve their selectivity. High depressant dosages reduce froth stability owing to the removal of naturally floatable gangue (such as talc) from the froth phase [9]. Frothers aid bubble formation and froth stabilisation. They increase froth stability by reducing the surface tension of the gas–liquid interface [22]. An increase in frother dosage increases froth stability and results in higher solids and water recoveries. Furthermore, frothers aid with gas dispersion and reduce bubble coalescence in the pulp phase [22]. The amount of entrained material is related to the water recovered [23,24,25], the state of the suspended solid particles in the pulp phase and drainage in the froth phase. Entrainment is directly related to the amount of water recovered from the froth phase [26,27,28], and as the ionic strength increases, the entrainability increases.
6. Froth Stability Measurements
Flotation recovery and concentrate grade are typically what are referred to when inferring flotation performance. Froth stability measurements aid the quantification and understanding of flotation performance.
Numerous methods have been proposed and used to measure froth stability, and these include measuring velocity of the froth, the rate of bursting of the froth and the solids loaded onto air bubble lamellae [29]. Non-overflowing and overflowing systems can be used for assessing froth stability. Overflowing systems are preferred over non-overlying systems, because they take into consideration continuous systems, which is what most industrial operations are [30].
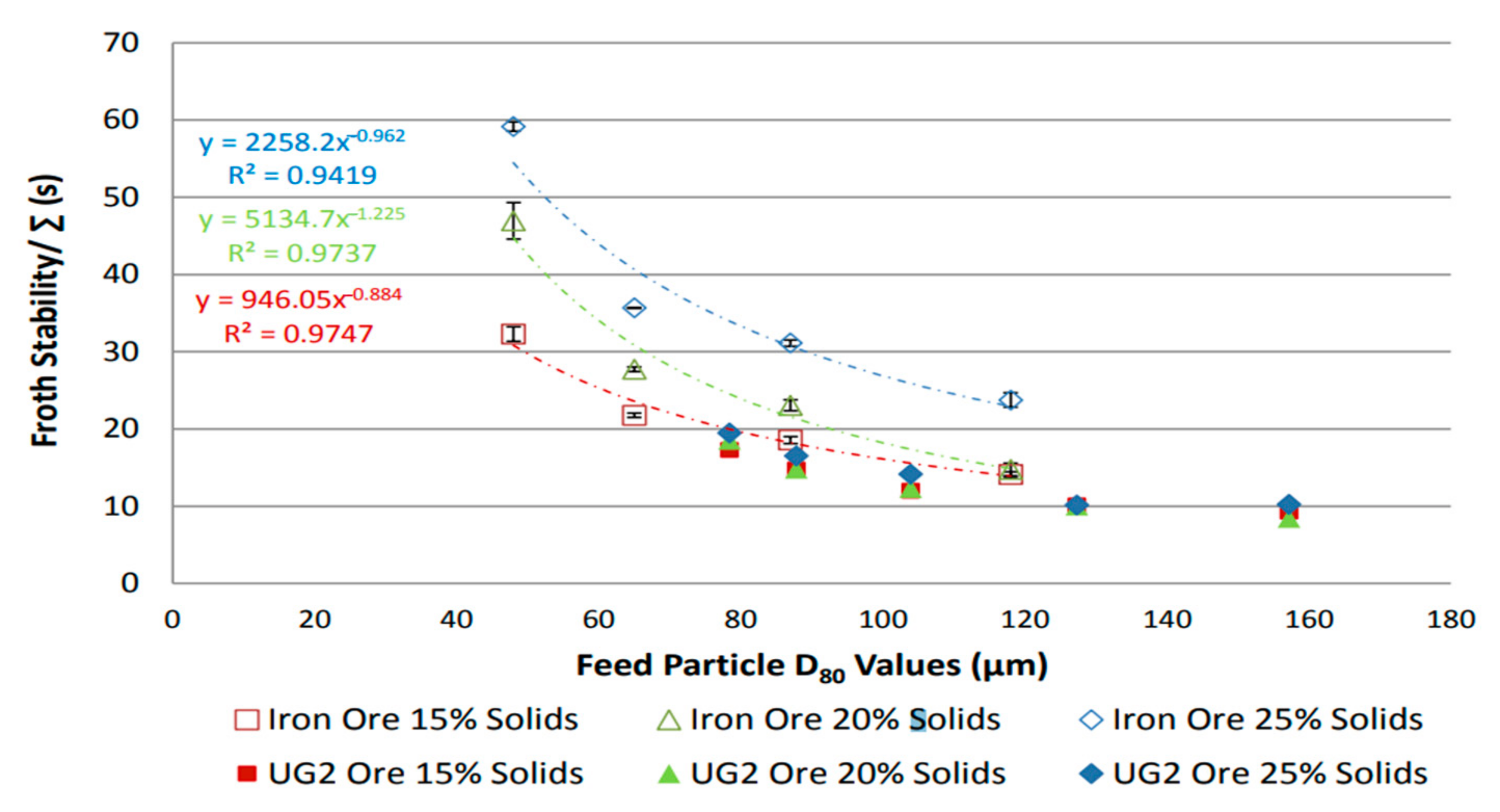
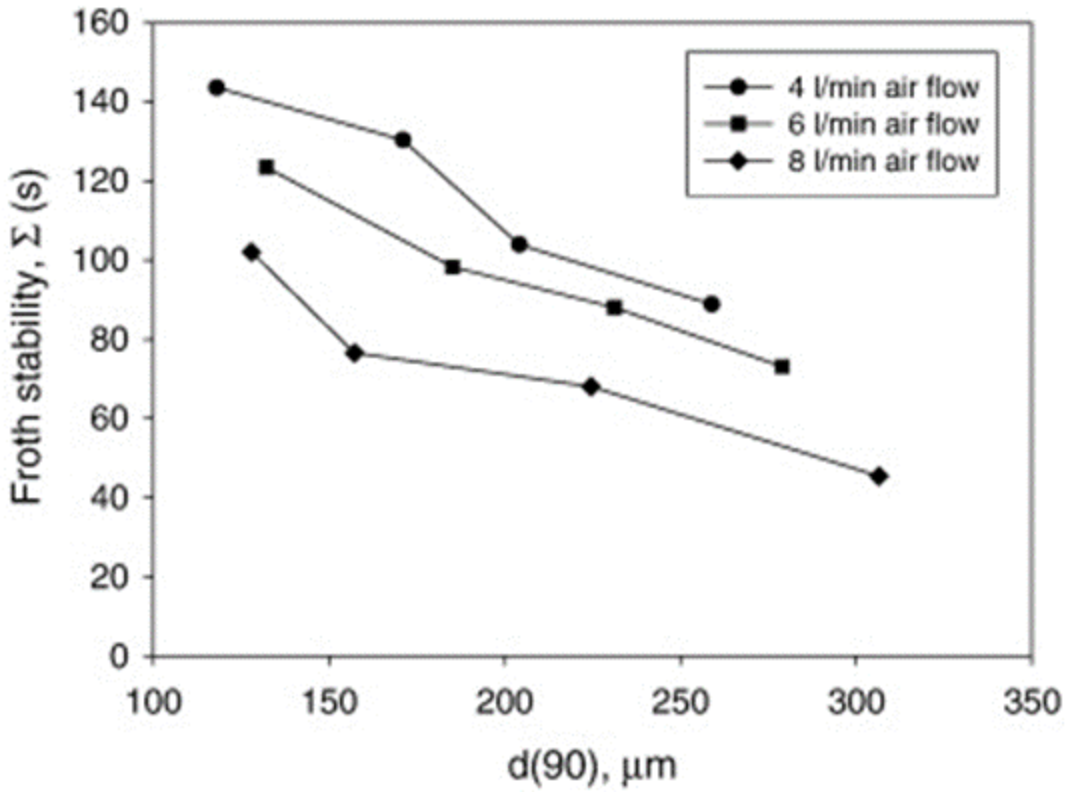
Ore mineralogy effects on froth stability were assessed by comparing two different ores, UG2 and iron ore [31], and dynamic froth stability was reported to increase exponentially with decreasing particle size, as shown in Figure 2. This is consistent with literature findings [32], which reported an inverse relationship between particle size and froth stability. Figure 3 shows that for a given particle distribution, the dynamic froth stability decreases as the airflow rate increases [20]. Furthermore, similar to Figure 2 and Figure 3 shows a decrease in dynamic froth stability as the feed particle size increases, thus implying that increasing the milling time and generally producing finer feed increases the dynamic froth stability. Froth height variations with time at different aeration rates illustrated these finding further [20].
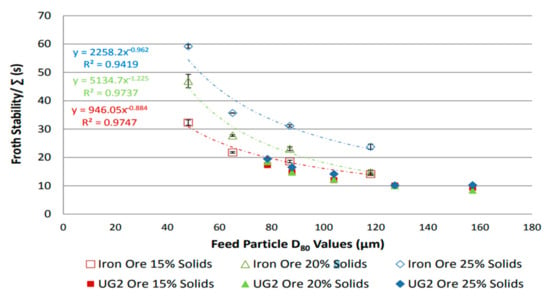
Figure 2.
Dynamic froth stability as function of feed particle size for iron ore and UG2 [31].
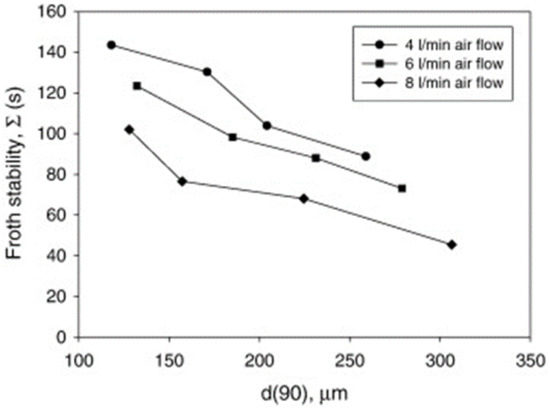
Figure 3.
Dynamic froth stability variations as a function of d(90) particle size for different flow rates [20].
Numerous studies have been conducted that relate the destabilizing effects of coarse particles and stabilizing effects of fine particles on froth stability [20,32,33].
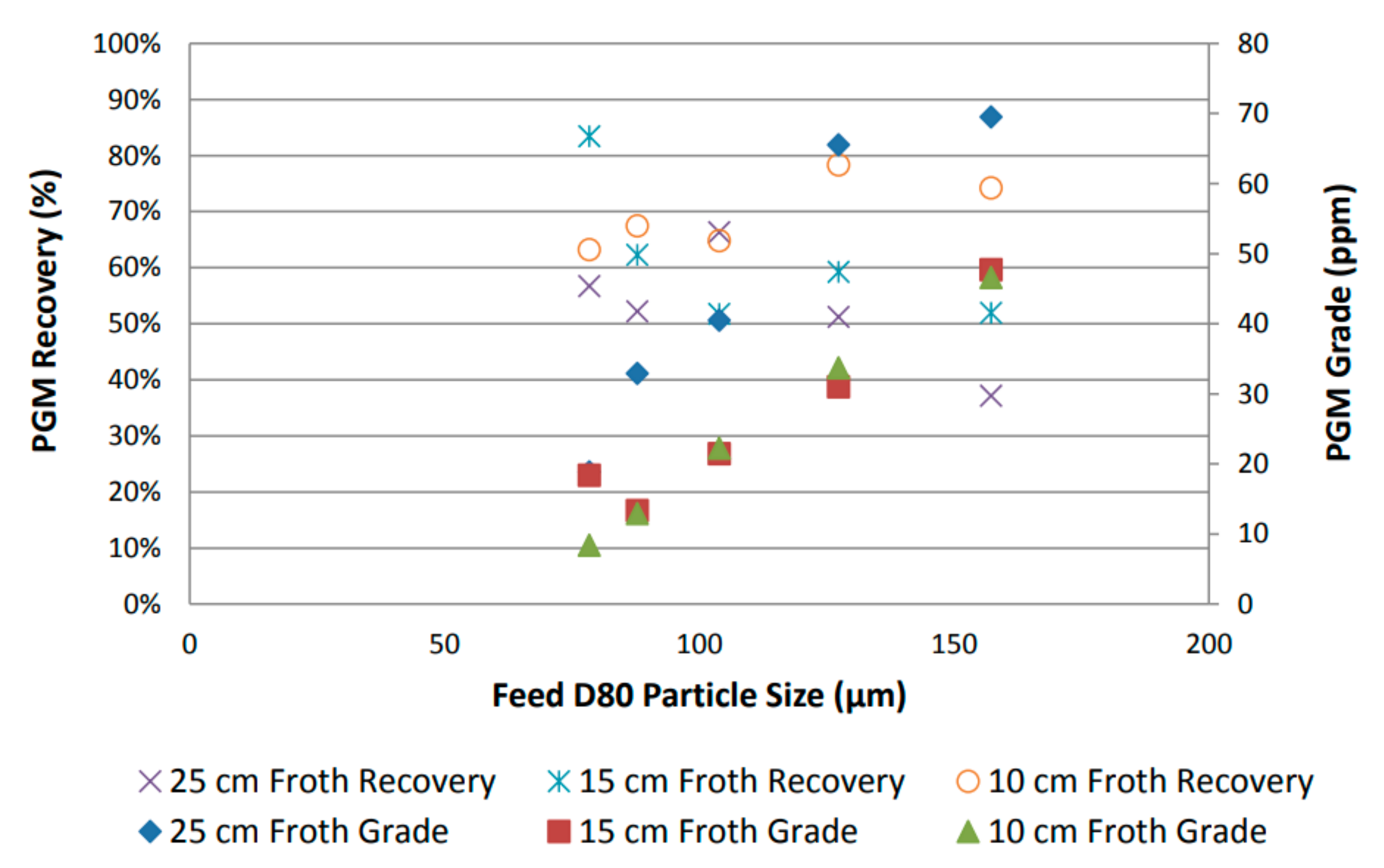
By using grade and desired mineral recovery as performance proxies, the overall flotation performance was assessed by plotting recovery as a function of feed particle size, as shown in Figure 4 [31]. An increase in PGM grade was observed with increasing particle size, and a reduction in PGM recovery was observed with an increase in froth height. Furthermore, a general increase in PGM recovery was observed with a decrease in feed particle size [31].
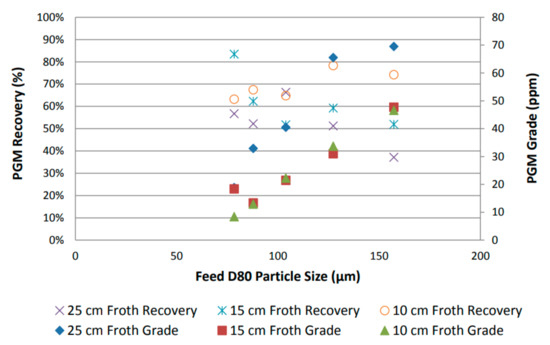
Figure 4.
PGM recovery and grade variation as function of feed particle size for UG2 [31].
Increasing conditioning time of the collector causes a decrease in froth stability, solid concentrate and water recovery. Flotation systems must exhibit optimum froth stability to attain optimum flotation performance; hence, an in-depth understanding and knowledge of the numerous factors affecting the system is paramount for mineral processing.
7. Future Work
Considering the discussed literature review and the ongoing research on froth flotation, future work must consider investigating the impact of ore feed grade on froth stability, entrainment, and flotation performance under varying water quality. The overarching aim of future work is the development of a relationship through which flotation performance may be predicted if the ore feed grade and water quality are known.
Author Contributions
Conceptualization, K.C.C.; methodology, K.C.C. and M.S.M.; investigation, M.N.; writing-original draft and preparation, M.N.; writing-review and editing, K.C.C., M.S.M., M.N.; supervision, K.C.C. and M.S.M.; funding acquisition, K.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the National Research Foundation (NRF) of South Africa (Grant number 123591). Any opinions, findings, conclusions or recommendations expressed in any publication generated by NRF supported research is that of the authors and the NRF accepts no liability. The authors wish to acknowledge the University of Cape Town (UCT) for providing funding assistance to support this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dzingai, M.; Manono, M.; Corin, K. Simulating the effect of water recirculation on flotation through ion-spiking: Effect of Ca2+ and Mg2+. Minerals 2020, 10, 1033. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Bradshaw, D.J. Synergistic Effects between Thiol Collectors Used in the Flotation Of Pyrite; University of Cape Town: Cape Town, South Africa, 1997. [Google Scholar]
- Melo, F.; Laskowski, J.S. Fundamental properties of flotation frothers and their effect on flotation. Miner. Eng. 2006, 19, 766–773. [Google Scholar] [CrossRef]
- Bradshaw, D.; Harris, P.J.; O’Connor, C. Synergistic interactions between reagents in sulphide flotation. J. South. Afr. Inst. Min. Metall. 1998, 98, 189–193. [Google Scholar]
- Davis, F.; Hyatt, D.; Cox, C. Environmental Problems of Flotation Reagents in Mineral Processing Plant Tailings Water Mineral Processing Plant Tailings Water; US Department of the Interior: Washington, DC, USA, 1975.
- Wiese, J.G. Investigating Depressant Behaviour in the Flotation of Selected Merensky Ores; University of Cape Town: Cape Town, South Africa, 2009. [Google Scholar]
- Bradshaw, D.; O’Connor, C.; Harris, P. The Effect of Collectors and Their Interactions with Depressants on the Behaviour of the Froth Phase in Flotation; The University Of Queensland: Brisbane, Australia, 2005. [Google Scholar]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Goodall, C.M. The Effects of Flotation Variables on the Bubble Size, Mixing Characteristics and Froth Behaviour in Column Flotation Cells; University of Cape Town: Cape Town, South Africa, 1993. [Google Scholar]
- Laskowski, J.; Woodburn, E.T. Frothing in Flotation II: Recent Advances in Coal Processing; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 1998.
- Klimpel, R.; Isherwood, S. Some industrial implications of changing frother chemical structure. Int. J. Miner. Process. 1991, 33, 369–381. [Google Scholar] [CrossRef]
- Smith, P.; Warren, L. Entrainment of particles into flotation froths. Miner. Procesing Extr. Metall. Rev. 1989, 5, 123–145. [Google Scholar]
- Yianatos, J.; Finch, J.; Laplante, A. Selectivity in column flotation froths. Int. J. Miner. Process. 1988, 23, 279–292. [Google Scholar] [CrossRef]
- Ventura-Medina, E.; Cilliers, J.J. A model to describe flotation performance based on physics of foams and froth image analysis. Int. J. Miner. Process. 2002, 67, 79–99. [Google Scholar] [CrossRef]
- Harris, P. Frothing Phenomena and Frothers. In Principles of Flotation; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1982; pp. 237–250. [Google Scholar]
- Wiese, J.; Harris, P.; Bradshaw, D. The response of sulphide and gangue minerals in selected Merensky ores to increased depressant dosages. Miner. Eng. 2007, 20, 986–995. [Google Scholar] [CrossRef]
- Subrahmanyam, T.; Forssberg, E. Froth stability, particle entrainment and drainage in flotation—A review. Int. J. Miner. Process. 1988, 23, 33–53. [Google Scholar]
- Aktas, Z.; Cilliers, J.J.; Banford, A.W. Dynamic froth stability: Particle size, airflow rate and conditioning time effects. Int. J. Miner. Process. 2008, 87, 65–71. [Google Scholar] [CrossRef]
- Napier-Munn, T. Preface to 7th Edition. In Wills’ Mineral Processing Technology, 7th ed.; Wills, B.A., Napier-Munn, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Zanin, M. An investigation into the effect of water quality on froth stability. Adv. Powder Technol. 2012, 23, 493–497. [Google Scholar] [CrossRef]
- Ekmekçi, Z.; Bradshaw, D.; Allison, S.; Harris, P. Effects of frother type and froth height on the flotation behaviour of chromite in UG2 ore. Miner. Eng. 2003, 16, 941–949. [Google Scholar] [CrossRef]
- Yang, X.-S.; Aldrich, C. Effects of impeller speed and aeration rate on flotation performance of sulphide ore. Trans. Nonferrous Met. Soc. China 2006, 16, 185–190. [Google Scholar]
- Boylu, F.; Laskowski, J.S. Rate of water transfer to flotation froth in the flotation of low-rank coal that also requires the use of oily collector. Int. J. Miner. Process. 2007, 83, 125–131. [Google Scholar]
- Neethling, S.J.; Cilliers, J.J. The entrainment factor in froth flotation: Model for particle size and other operating parameter effects. Int. J. Miner. Process. 2009, 93, 141–148. [Google Scholar] [CrossRef]
- Engelbrecht, J.A.; Woodburn, E.T. The Effects of Froth Height, Aeration Rate and Gas Precipitation on Flotation. J. South Afr. Inst. Min. Metall. 1975, 76, 125–132. [Google Scholar]
- Zheng, X.; Johnson, N.W.; Franzidis, J.P. Modelling of entrainment in industrial flotation cells: Water recovery and degree of entrainment. Miner. Eng. 2006, 19, 1191–1203. [Google Scholar] [CrossRef]
- Vera, M.; Mathe, Z.; Franzidis, J.-P.; Harris, M.; Manlapig, E.; O’Connor, C. The modelling of froth zone recovery in batch and continuously operated laboratory flotation cells. Int. J. Miner. Process. 2002, 64, 135–151. [Google Scholar]
- Barbian, N.; Ventura-Medina, E.; Cilliers, J. Dynamic froth stability in froth flotation. Miner. Eng. 2003, 16, 1111–1116. [Google Scholar] [CrossRef]
- Chidzanira, T. Investigation of the Effect of Particle Size on Froth Stability; University of Cape Town: Cape Town, South Africa, 2016. [Google Scholar]
- Ip, S.; Wang, S.; Toguri, J. Aluminum foam stabilization by solid particles. Can. Metall. Q. 1999, 38, 81–92. [Google Scholar] [CrossRef]
- Johansson, G.; Pugh, R. The influence of particle size and hydrophobicity on the stability of mineralized froths. Int. J. Miner. Process. 1992, 34, 1–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).