The Macrophytic Vegetation of River Ethiope at the Umuaja Ukwani Local Government Area of Delta State, Nigeria †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collectio
2.2. Physicochemical Parameters
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antti, K. Aquatic macrophytes in status assessment and monitoring of boreal lakes. Jyvaswa Stud. Biol. Environ. Sci. 2012, 254, 1–151. [Google Scholar]
- Brum, P.R.; Prast, A.E.; De Assis Esteves, F. Changes in the allocation of some chemical compounds in structures of Oryza glumaepatula (Steud) in an Amazonian Lake subjected to an anthropic impact (Lake Batata, Porto Trombetas). Hydrobiologia 2006, 570, 27–33. [Google Scholar] [CrossRef]
- Chambers, P.A.; Lacoul, P.; Murphy, K.J.; Thomaz, S.M. Global diversity of aquatic ecosystem in freshwater. J. Hydrobiol. 2008, 595, 9–26. [Google Scholar] [CrossRef]
- Igor, Z.; Mateja, P.; Alenka, G. Environmental conditions and macrophytes of karst ponds. Pol. J. Environ. Stud. 2012, 21, 1911–1920. [Google Scholar]
- Marina, T.; Dragana, P.; Aleksandar, O. Temporaerbial and habitat distribution of macrophytes in lowland eutrophic reservoir gruza in serbia. Period. Biol. 2015, 117, 67–73. [Google Scholar]
- Okayi, R.G.; Daku, V.; Mbata, F.U. Some aquatic macrophytes and water quality parameters of river guma, benue, Nigeria. Niger. J. Fish. Aquacultre 2013, 1, 25–30. [Google Scholar]
- Onaindia, M.; Amezaga, I.; Garbisu, C.; Garcia-Bikuna, B. Aquatic macrophytes as biological indicators of environmental conditions of rivers in north-eastern span. Int. J. Limnol. 2005, 41, 175–182. [Google Scholar] [CrossRef]
- Oyedeji, A.A.; Abowei, J.F.N. The classification, distribution, control and economic importance of aquatic plants. Int. J. Fish. Aquat. Sci. 2012, 1, 118–128. [Google Scholar]
- Shah, M.; Hashmi, H.M.; Ghumman, A.R.; Zeeshan. Performance assessment of aquatic macrophytes for treatment of municipal wastewater. J. S. Afr. Inst. Civ. Eng. 2015, 57, 18–25. [Google Scholar] [CrossRef]

| Parameters | January | February | March |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Temperature (°C) | 26.5 ± 0.602 | 27.2 ± 0.19 | 26.6 ± 0.16 |
| Conductivity (ms/m) | 133.9 ± 0.637 | 110.06 ± 0.34 | 114.2 ± 0.42 |
| Alkalinity (mg/L) | 73.33 ± 26.26 | 58.33 ± 19.29 | 59.00 ± 17.45 |
| pH | 7.93 ± 0.367 | 4.25 ± 0.39 | 5.76 ± 0.25 |
| Calcium (mg/L) | 7.46 ± 0.754 | 13.00 ± 0.18 | 9.07 ± 1.36 |
| Total Hardness (mg/L) | 10.00 ± 1.63 | 13.74 ± 0.81 | 14.53 ± 1.80 |
| Magnesium (mg/L) | 2.54 ± 0.88 | 0.74 ± 0.63 | 5.46 ± 0.44 |
| DO (mg/L) | 4.13 ± 0.94 | 9.73 ± 2.97 | 8.26 ± 1.00 |
| BOD (mg/L) | 1.16 ± 0.19 | 3.00 ± 0.38 | 5.67 ± 0.57 |
| Sulphate (mg/L) | 190.63 ± 2.81 | 180.4 ± 0.92 | 165.46 ± 4.24 |
| Transparency (%) | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Chloride (mg/L) | −0.04 ± 0.039 | −0.12 ± 0.027 | −0.10 ± 0.01 |
| Nitrate (mg/L) | 7.60 ± 0.66 | 10.13 ± 0.66 | 7.33 ± 0.57 |
| Phosphate (mg/L) | 0.14 ± 0.02 | 0.40 ± 0.37 | 0.76 ± 0.48 |
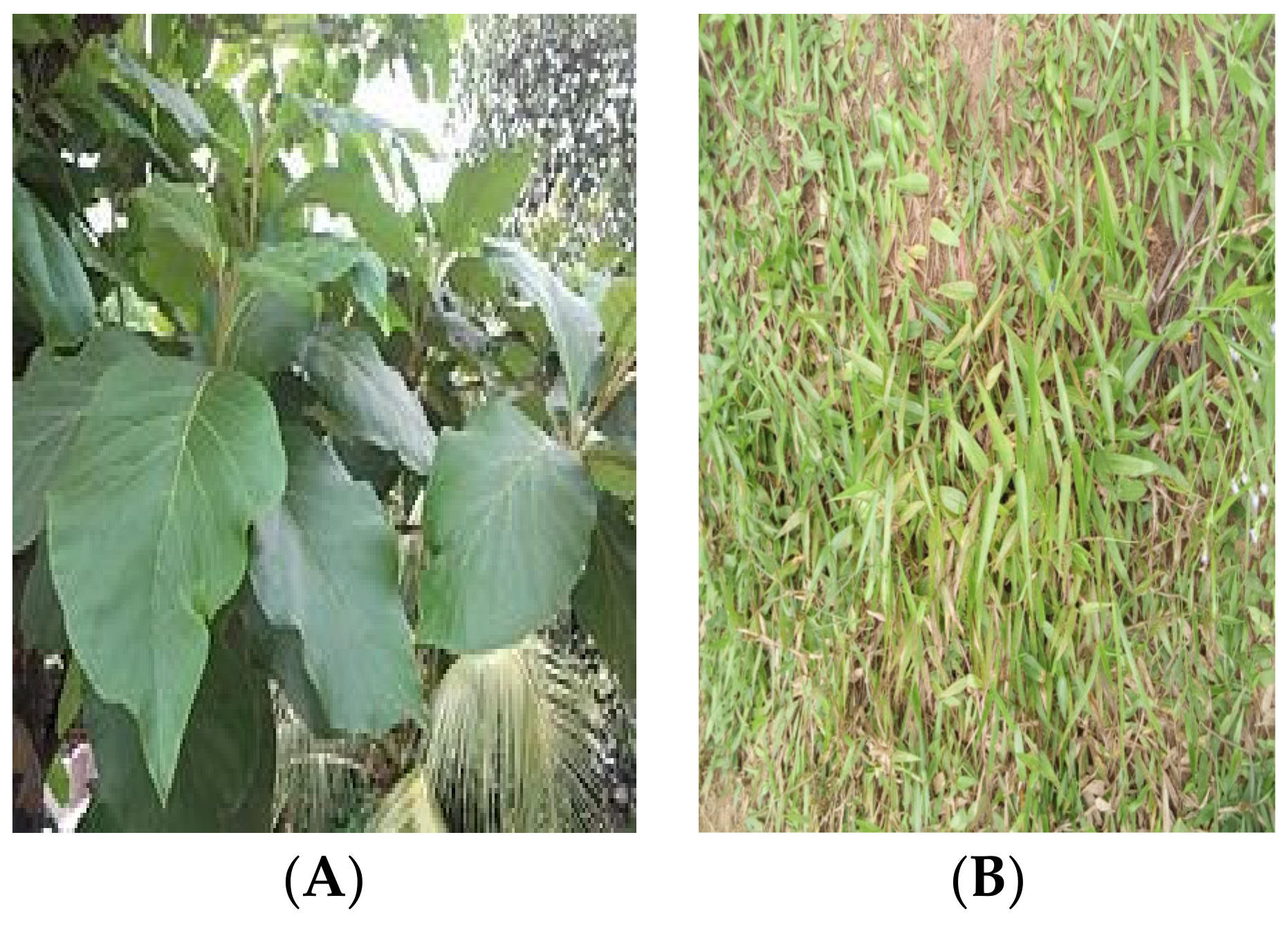
| Botanical Name | Family Name | Life Form | Abundance | Ecological Status |
|---|---|---|---|---|
| Bumbus vulgaris | Poaceae | Embankment | 5 | Abundance |
| Cieba pentandra | Malvaceae | Embankment | 1 | Rare |
| carex spp | Cyperaceae | Embankment | 4 | Abundance |
| Elaeis guineensis | Areceae | Embankment | 4 | Abundance |
| Azonopus compressus | Poaceae | Embankment | 4 | Abundance |
| Acanthus montanus | Acanthaceae | Embankment | 2 | Rare |
| Tectona grandis | Lamiaceae | Embankment | 4 | Abundance |
| Calopogonium mucunoids | Fabaceae | Embankment | 2 | Rare |
| Anacardium occidentale | Anacardiaceae | Embankment | 2 | Rare |
| Alternanthera sessilis | Amaranthaceae | Embankment | 3 | Abundance |
| Ipomoea aquatica | Convolvulaceae | Emergent | 2 | Rare |
| Leersia hexandra | Convolvulaceae | Embankment | 3 | Abundance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onochie, P.; Amarie, E.-O. The Macrophytic Vegetation of River Ethiope at the Umuaja Ukwani Local Government Area of Delta State, Nigeria. Eng. Proc. 2023, 37, 103. https://doi.org/10.3390/ECP2023-14650
Onochie P, Amarie E-O. The Macrophytic Vegetation of River Ethiope at the Umuaja Ukwani Local Government Area of Delta State, Nigeria. Engineering Proceedings. 2023; 37(1):103. https://doi.org/10.3390/ECP2023-14650
Chicago/Turabian StyleOnochie, Prosper, and Elohor-Oghene Amarie. 2023. "The Macrophytic Vegetation of River Ethiope at the Umuaja Ukwani Local Government Area of Delta State, Nigeria" Engineering Proceedings 37, no. 1: 103. https://doi.org/10.3390/ECP2023-14650
APA StyleOnochie, P., & Amarie, E.-O. (2023). The Macrophytic Vegetation of River Ethiope at the Umuaja Ukwani Local Government Area of Delta State, Nigeria. Engineering Proceedings, 37(1), 103. https://doi.org/10.3390/ECP2023-14650






