Abstract
Cow milk is more allergenic than milk from other species, and therefore the adulteration of ewe or goat milk with cow milk can pose a serious threat to consumers. In this work, a silicon-based photonic immunosensor, which includes two U-shaped Mach–Zehnder Interferometers (MZIs), was employed for the detection of ewe and goat milk adulteration with cow milk through the immunochemical determination of the milk. The method was fast and sensitive with a detection limit of 0.04 μg/mL bovine k-casein (which corresponds to approximately 0.06% cow milk) in ewe or goat milk, respectively, and with a total assay time of 12 min.
1. Introduction
Milk is a highly nutritious product consumed by millions of people worldwide, and its authenticity and safety are crucial for public health. The adulteration of ewe and goat milks with cow milk reduces their nutritional value and exposes consumers to potential health risks, especially to allergies and digestive problems for individuals who are intolerant to cow milk [1]. The accurate identification of adulterants in milk is essential for ensuring the safety and quality of milk products, protecting public health, regulating the milk industry and promoting food safety. For that reason, the European Commission (EC) has set a maximum acceptable content of cow milk in dairy products from other species of 1% (v/v) [2]. To detect milk adulteration, several methods have been employed such as chromatographic, molecular and immunological ones, which, however, require trained personnel and cannot be performed at the point of need [3,4,5,6]. On the other hand, biosensors can provide fast and quantitative on-site determinations and are therefore widely used in the field of food analysis [7,8].
In this work, we employed a newly introduced immersible silicon chip accommodating two U-shaped silicon nitride waveguides formatted as Mach–Zehnder interferometers (MZIs) [9]. The sensing arms of the two MZI sensors were modified with bovine k-casein and bovine serum albumin, respectively, in order to serve as working and reference sensors. The two MZI waveguides allow light in- and out-coupling from the same chip side and thus the chip has the ability to be immersed into the sample. This feature makes the instrumentation of the proposed immunosensor simpler than other sensing systems that require external pumps and microfluidics. The simplicity of the instrumentation, its small size, along with the short assay duration and high assay sensitivity make the developed biosensing system ideal for the on-site detection of ewe or goat milk adulteration.
2. Materials and Methods
2.1. Reagents
Bovine serum albumin (BSA) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Bovine k-casein, goat anti-rabbit IgG antibody (secondary antibody) and 3-aminopropyltriethoxysilane (APTES) were purchased from Sigma-Aldrich (Darmstadt, Germany). The anti-bovine k-casein rabbit antiserum was in-house developed, as described in a previous work [10]. All other chemicals and reagents were obtained from Merck (Darmstadt, Germany). The water used throughout the study was doubly distilled. Pasteurized ewe (1.7% fat) and goat milk (3.5% fat) (OLYMPOS FOODS S.A., Larissa, Greece) were obtained from local supermarkets.
2.2. Biosensing Principle
In this work, a label-free optical immunosensor based on Mach–Zehnder Interferometry was used to determine milk adulteration through a competitive immunoassay for the determination of bovine k-casein in ewe and goat milk. In an MZI, the incoming light is split into two arms, the sensing and the reference one, and when the two arms combine again, an interference spectrum is created in the output. On the sensing arm of the MZI, the SiO2 cladding layer is removed over the sensing window, which allows the analyte to interact with the waveguided photons. The biomolecular reactions that take place on the sensing arm change the refractive index on the surface of the waveguide, causing a phase shift of the interference spectrum, thus providing a way to monitor the adlayer growth on the sensing arm. More specifically, in the developed immunosensor, there are two MZI sensors on the same chip side in order to allow the chip immersion in the sample. The biosensing system includes a broad-band white light source and an external spectrophotometer that records the transmission spectrum of both MZIs. The spectrum is subjected to Fast Fourier Transform to distinguish the phase shift of the two MZIs, due to interactions taking place on them, and provide real time monitoring.
2.3. Chemical and Biological Functionalization
The chips were cleaned and hydrophilized through piranha treatment followed by aminosilanization. More specifically, the chips were immersed in H2SO4:H2O2 solution (1:1) for 20 min, washed with H2O and immersed again in 2% (v/v) APTES solution in water for 20 min. After washing with H2O, the chips were heated at 120 °C for 20 min. Then, the open windows of the working and reference MZIs were spotted using a microarray spotter with bovine k-casein (50 μg/mL) and BSA (50 μg/mL), respectively. After the 1 h incubation of the chips in a 70% humidity chamber, they were washed and blocked by immersion in a 1% BSA solution in 0.1 M NaHCO3 solution, pH 8.5, for 1 h. Then, the chips were washed with 0.05 M PBS, dried under a nitrogen stream and kept in a desiccator at room temperature until use.
2.4. Immunoassay
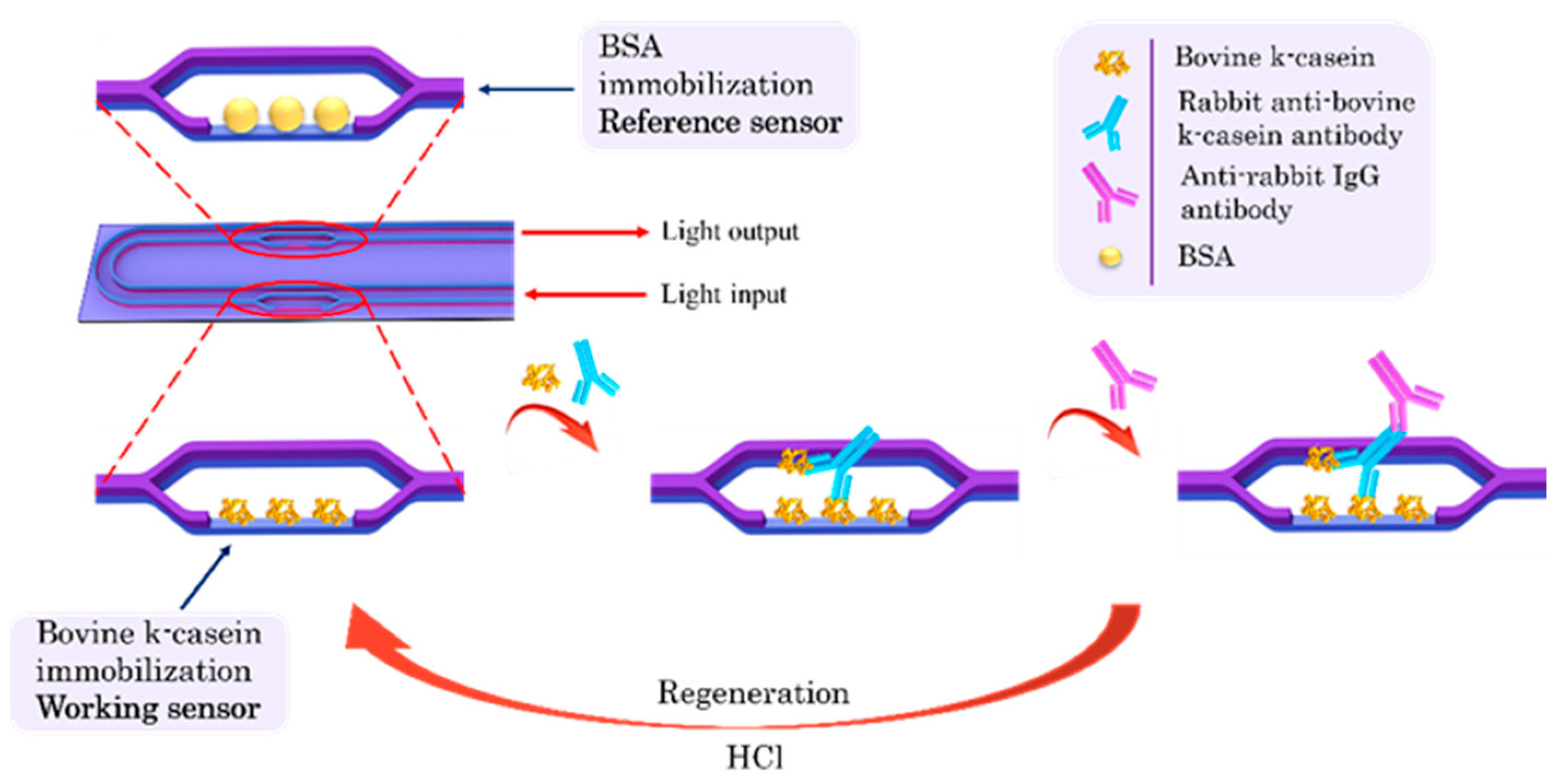
To detect the bovine k-casein in ewe/goat milk, the competitive immunoassay principle was followed. A schematic of the immunoassay steps is depicted in Figure 1. For the assay, 1:1 (v/v) mixtures of bovine k-casein calibrators prepared in assay buffer or in 50-times-diluted ewe or goat milk with the rabbit anti-bovine k-casein antibody were preincubated for 1 h at room temperature. The chip was equilibrated by immersion in assay buffer (0.01 M PBS, pH 7.4 containing 0.5% (w/v) BSA and 0.05% Tween® 20 (v/v) or in 100-times-diluted ewe or goat milk. Once a stable baseline was achieved, the chip was immersed in a microtiter well containing the preincubated mixture of the calibrator with the antibody for 5 min. Then, the chip was immersed for 2 min in assay buffer to remove the unbound immunoreagents and then immersed in a secondary anti-rabbit IgG antibody solution (10 μg/mL) in assay buffer for 5 min. After that, the chip was regenerated through immersion for 2 min in a 50 mM HCl solution followed by equilibration in assay buffer. The net phase shift (signal) was calculated by determining the difference in the phase shift of the working sensor from that of the reference sensor due to the reaction of the anti-rabbit IgG antibody. The calibration curve was created by plotting the % ratios of the bovine k-casein calibrator signals (Sx), with respect to the zero calibrator signal (S0).
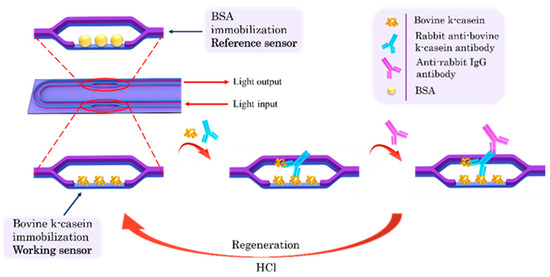
Figure 1.
Schematic of the chip and of immunoassay steps for detection of bovine k-casein in milk from other species with the MZI photonic sensors.
3. Results and Discussion
3.1. Assay Optimization
In order to develop the immunoassay for ewe/goat milk adulteration with bovine milk, several parameters were optimized, including the concentration of immobilized bovine k-casein, the anti-k-casein antibody concentration, the preincubation time and the matrix effect. At first, it was found that adequate signal response, along with high sensitivity was achieved when the bovine k-casein concentration used for the spotting of the chip surface was equal to or higher than 50 μg/mL, employing a 50 times anti-k-casein antiserum dilution. Furthermore, different preincubation times were employed (15, 30 and 60 min) and it was found that a 1 h preincubation time significantly increased the assay detection sensitivity. These optimization experiments were performed using bovine k-casein calibrators prepared in assay buffer (0.01 M PBS, pH 7.4 containing 0.5% (w/v) BSA and 0.05% Tween® 20 (v/v). Regarding bovine k-casein detection in ewe or goat milk, it was found that using a 50 times milk dilution provided identical calibration curves to those obtained with calibrators prepared in assay buffer. In order to investigate whether the chip could be reused, we immersed the chip in different solutions (hydrochloride solution, sodium hydroxide) and it was found that the immersion of the chip in 50 mM hydrochloride solution for 2 min resulted in the complete regeneration of the chip, allowing it to be reused at least 12 times.
3.2. Analytical Characteristics
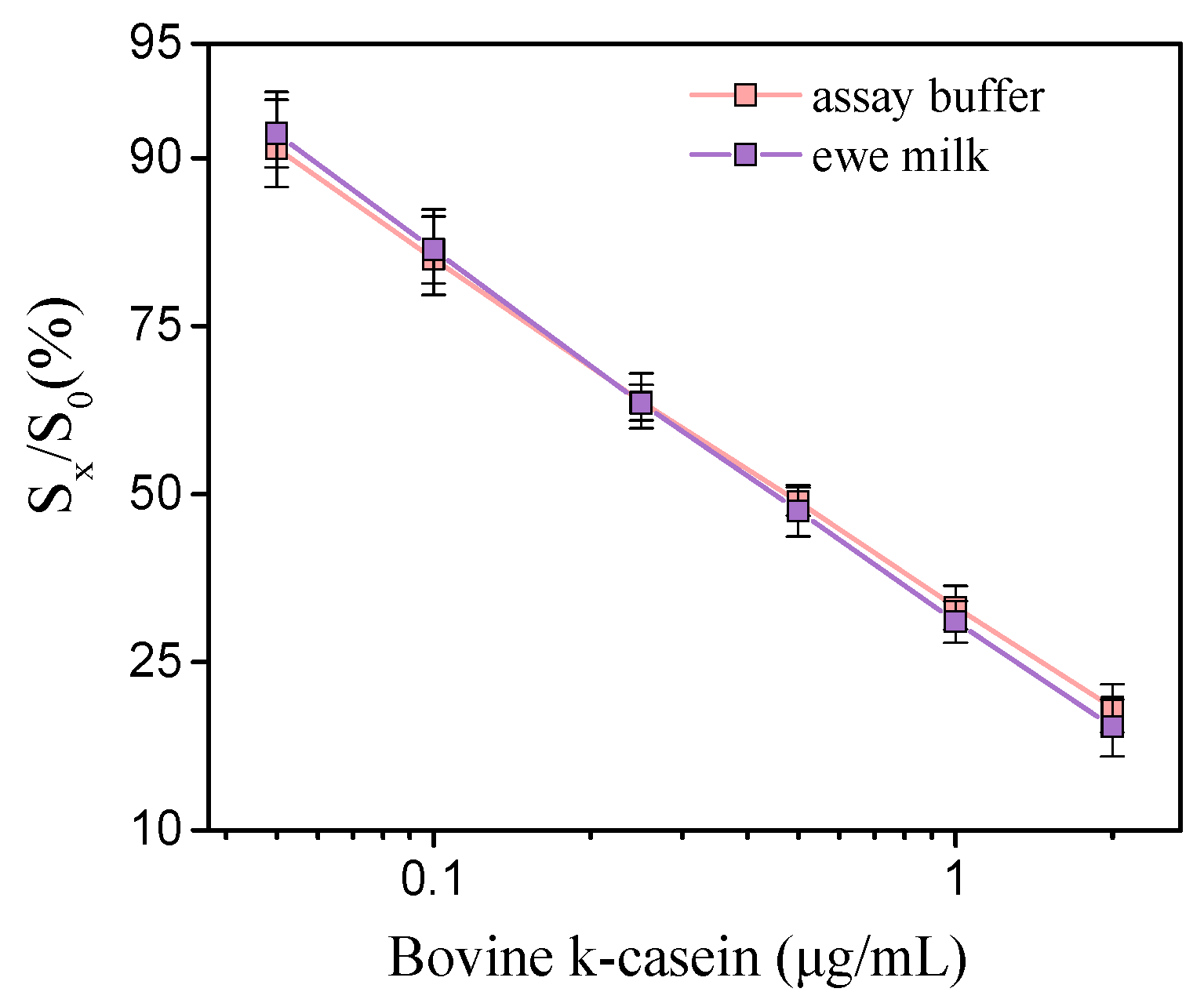
The calibration curves for bovine k-casein prepared either in assay buffer or 50-times-diluted ewe milk are presented in Figure 2. As is shown in the graph, the calibration curves prepared in assay buffer and in 50-time diluted milk were superimposed. The detection limit of the assay using calibrators in ewe or goat milk was calculated as the concentration corresponding to a signal equal to -3SD of the mean zero calibrator signals of 10 measurements and it was 0.04 μg/mL of bovine-k-casein, with a working range from 0.1 to 2 μg/mL. Based on the mean content of bovine milk in k-casein (3.4 g/L), it is calculated that the lowest detectable amount of cow milk in undiluted ewe or goat milk that can be detected with the method developed is less than 0.058%, which is well below the EU limit (1%) regarding the adulteration of ewe/goat milk with cow milk.
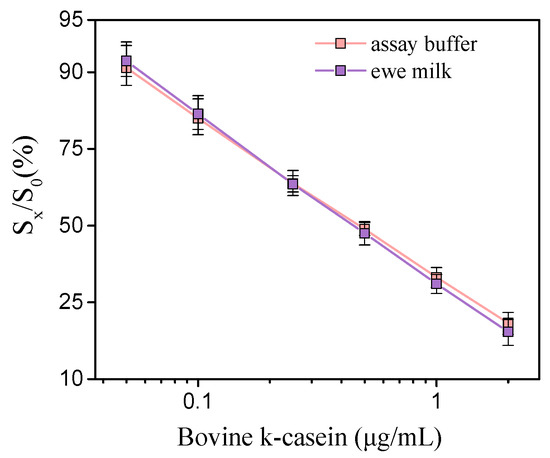
Figure 2.
Typical calibration curves of bovine k-casein (μg/mL) in assay buffer (pink line) and 50-times-diluted ewe milk (purple line). (Sx/S0)% represents the percent ratio of each calibrator signal (Sx) to the zero calibrator signal (S0). Each point is the mean value of 3 measurements ± SD.
The intra- and inter-assay coefficients of variation (CVs) were determined by measuring duplicates of different control samples, prepared through the spiking of appropriate amounts of bovine k-casein in ewe milk (final concentrations of 0.15, 0.3 and 0.9 μg/mL), during the same day and during 5 random days in 1 month, and were 4% and 6.5%, respectively, indicating the high repeatability of the assay.
The accuracy of the assay was determined through recovery experiments with samples of ewe and goat milk spiked with three different concentration levels of bovine k-casein (0.4, 0.8 and 1.5 μg/mL). As presented in Table 1, the recovery values ranged from 94.6 to 107%, indicating the high accuracy of the proposed immunoassay.

Table 1.
% Recovery of known amounts of bovine k-casein spiked in ewe/goat milk.
4. Conclusions
In this work, an immersible biosensing platform to detect milk adulteration with cow milk in ewe and goat milk based on the detection of bovine k-casein was developed. A competitive immunoassay format was followed where the chip was first immersed in a mixture of 50-times-diluted ewe or goat milk with a rabbit anti-k-casein antibody solution for 5 min, followed by 5 min immersion in a secondary antibody solution. A limit of detection of 0.04 μg/mL in terms of bovine k-casein, which corresponds to approximately 0.06% cow milk in undiluted ewe/goat milk, was achieved with a total assay time of 12 min. Thus, the excellent analytical performance of the assay developed, combined with the portability of the sensing system makes this device suitable for the on-site monitoring of milk adulteration.
Author Contributions
Conceptualization, M.A., K.M., E.M., P.P., and S.K.; methodology, D.K., M.A., K.M., E.M., P.P. and S.K.; validation, D.K., M.A., P.P., and S.K.; formal analysis, D.K. and M.A.; investigation, D.K. and M.A.; resources, K.M., E.M., P.P. and S.K.; data curation, D.K. and M.A.; writing—original draft preparation, D.K. and M.A.; writing—review and editing, A.E., P.P. and S.K.; visualization, D.K. and M.A.; supervision, S.K.; project administration, S.K.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: Τ2ΕΔΚ-01934 FOODSENS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The relevant data are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Montgomery, H.; Haughey, S.A.; Elliott, C.T. Recent food safety and fraud issues within the dairy supply chain (2015–2019). Glob. Food Sec. 2020, 26, 100447. [Google Scholar] [CrossRef] [PubMed]
- European Commission EC 213/2001, Methods for the analysis and quality evaluation of milk and milk products. Off. J. Eur. Comm. 2001, 44, L37/31–L37/99.
- Nagraik, R.; Sharma, A.; Kumar, D.; Chawla, P.; Kumar, A.P. Milk adulterant detection: Conventional and biosensor based approaches: A review. Sens. Biosensing Res. 2021, 33, 100433. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Li, M.; Yang, Y.; Wang, Z.; Miao, J.; Zhao, Z.; Yang, J. Detection of the adulteration of camel milk powder with cow milk by ultra-high performance liquid chromatography (UPLC). Int. Dairy J. 2021, 121, 105117. [Google Scholar] [CrossRef]
- Song, H.; Xue, H.; Han, Y. Detection of cow’s milk in Shaanxi goat’s milk with an ELISA assay. Food Control 2011, 22, 883–887. [Google Scholar] [CrossRef]
- Vincent, D.; Elkins, A.; Condina, M.R.; Ezernieks, V.; Rochfort, S. Quantitation and Identification of Intact Major Milk Proteins for High-Throughput LC-ESI-Q-TOF MS Analyses. PLoS ONE 2016, 11, e0163471. [Google Scholar] [CrossRef] [PubMed]
- Nehra, M.; Lettieri, M.; Dilbaghi, N.; Kumar, S.; Marrazza, G. Nano-Biosensing Platforms for Detection of Cow’s Milk Allergens: An Overview. Sensors 2020, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Paliwal, A.; Bassi, M.; Tomar, M.; Gupta, V.; Gulati, S. Investigation of Adulteration in Milk using Surface Plasmon Resonance. ECS J. Solid State Sci. Technol. 2021, 10, 091004. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Makarona, E.; Salapatas, A.; Misiakos, K.; Synolaki, E.; Ioannidis, A.; Chatzipanagiotou, S.; Ritvos, M.A.; Pasternack, A.; Ritvos, O.; et al. Directly immersible silicon photonic probes: Application to rapid SARS-CoV-2 serological testing. Biosens. Bioelectron. 2022, 215, 114570. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.S.; Raptis, I.; Misiakos, K.; Livaniou, E.; Makarona, E.; Kakabakos, S. Rapid detection of mozzarella and feta cheese adulteration with cow milk through a silicon photonic immunosensor. Analyst 2021, 146, 529–537. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).