Biocompatible and Flexible Transparent Electrodes for Skin-Inspired Sensing †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Fabrication of the Transparent Electrode Platforms
2.3.1. Preparation of the Chitosan Membranes
2.3.2. Preparation of the Chi-LaA-AgNW Dispersions
2.3.3. Screen-Printing of the Chitosan Membranes
2.4. Electrochemical Assays
3. Results and Discussion
3.1. Fabrication of the Transparent Electrode Platforms
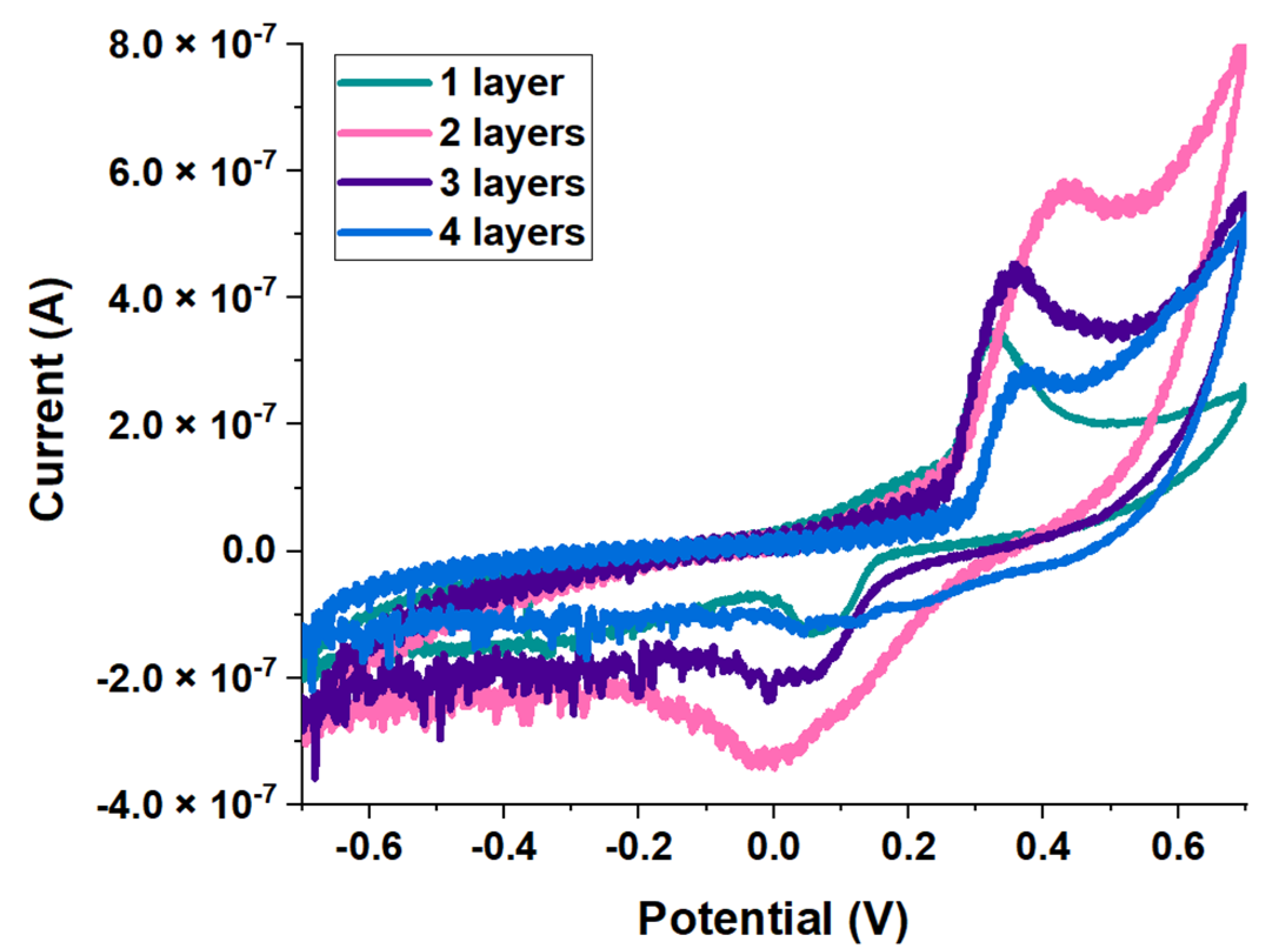
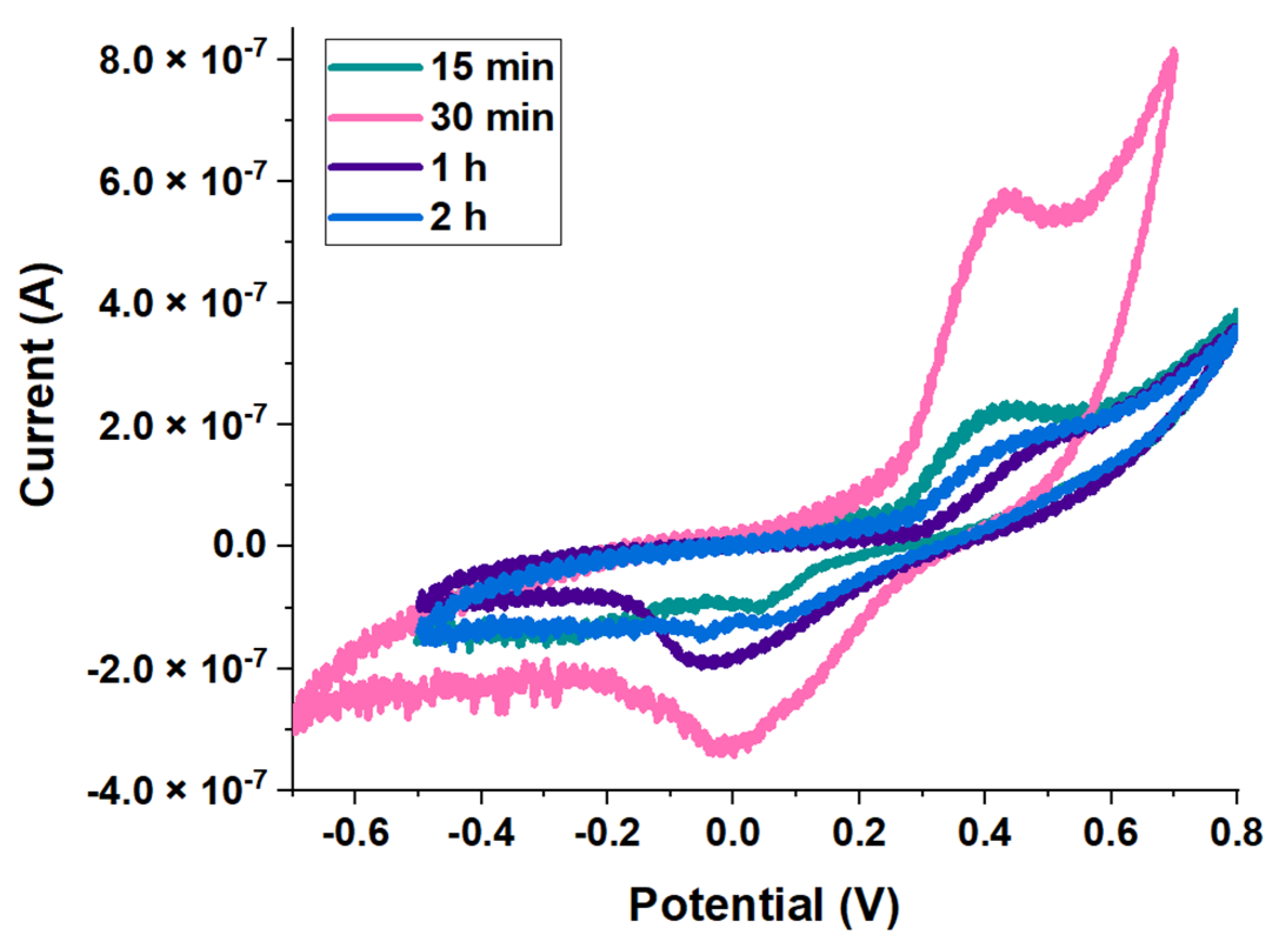
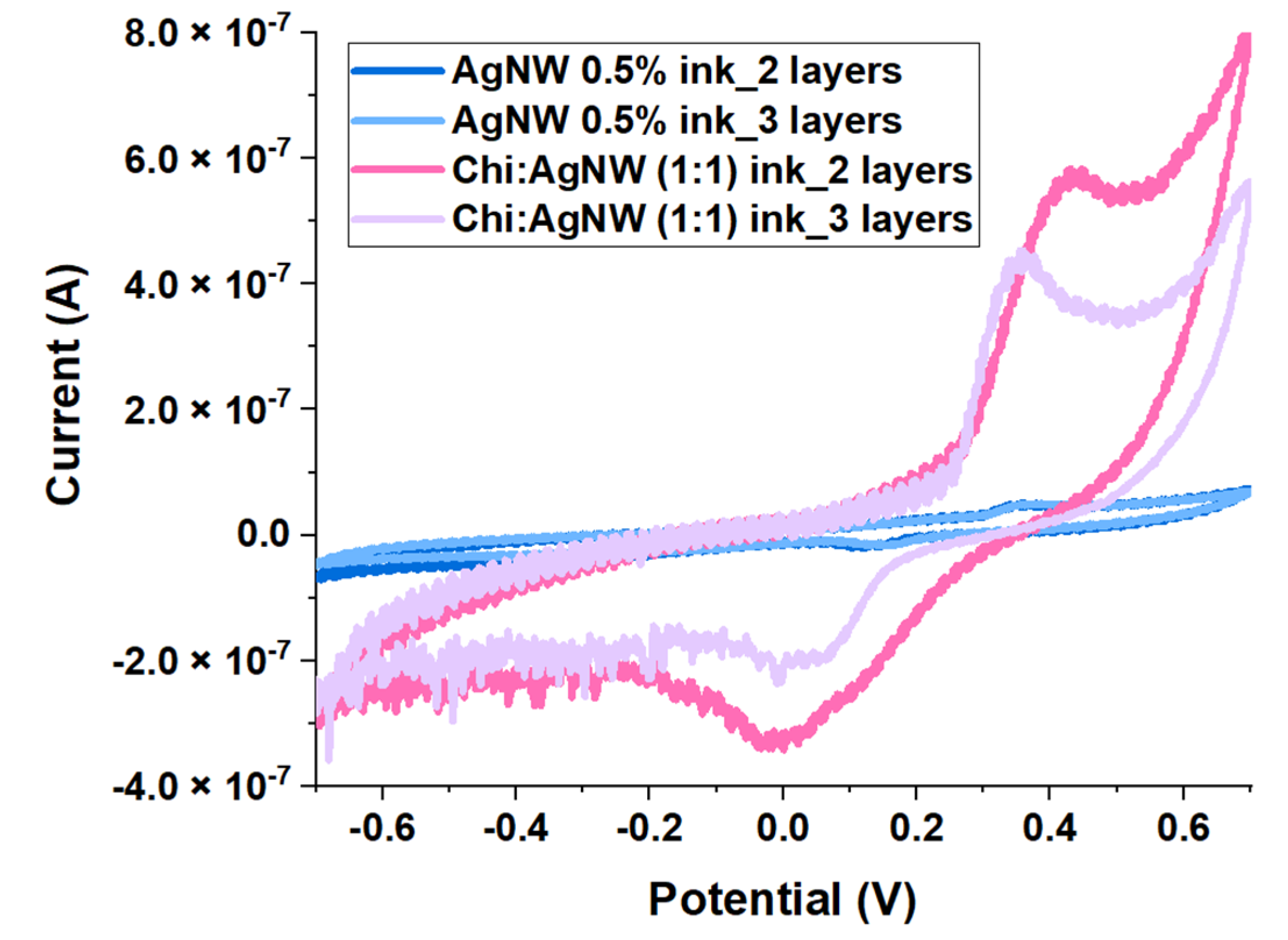
3.2. Electrochemical Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Wang, S.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z. Skin-Inspired Electronics: An Emerging Paradigm. Acc. Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Zhang, Y.; Wang, L.; Wei, C.; Lv, T.; Wang, Z.L.; Wu, Z. Sweat-Permeable, Biodegradable, Transparent and Self-Powered Chitosan-Based Electronic Skin with Ultrathin Elastic Gold Nanofibers. Adv. Funct. Mater. 2022, 32, 2112241. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Kang, P.-L.; Xin, W.; Yen, C.-S.; Hwang, L.-C.; Chen, C.-J.; Liu, J.-T.; Chang, S.J. Preparation and Evaluation of Chitosan Biocompatible Electronic Skin. Comput. Ind. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Lü, X.; Zhang, H.; Huang, Y.; Zhang, Y. A Proteomics Study to Explore the Role of Adsorbed Serum Proteins for PC12 Cell Adhesion and Growth on Chitosan and Collagen/Chitosan Surfaces. Regen. Biomater. 2018, 5, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, Y.; Qian, B.; Wang, J.; Xiao, F. One-Step Aqueous Fabrication of a Silver Nanowire Composite Transparent Conductive Film with High Uniformity and Stability. J. Mater. Chem. C 2020, 8, 4372–4384. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, S.; Yin, T.; Zhang, H.; Wang, C.; Wang, Y.; Li, Q.; Zhou, N.; Su, F.; Jiang, Z.; et al. Room-Temperature Nanojoining of Silver Nanowires by Graphene Oxide for Highly Conductive Flexible Transparent Electrodes. Nanotechnology 2022, 34, 045201. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Umar, M.; Seo, H.; Yim, J.H.; Kam, D.G.; Jeon, H.; Lee, S.; Kim, S. Biocompatible, Optically Transparent, Patterned, and Flexible Electrodes and Radio-Frequency Antennas Prepared from Silk Protein and Silver Nanowire Networks. RSC Adv. 2016, 7, 574–580. [Google Scholar] [CrossRef]
- Chen, Y.; Carmichael, R.S.; Carmichael, T.B. Patterned, Flexible, and Stretchable Silver Nanowire/Polymer Composite Films as Transparent Conductive Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 31210–31219. [Google Scholar] [CrossRef] [PubMed]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N.; Phisalaphong, M. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin as A Multifunctional Biopolymer Composite Film. Molecules 2020, 25, 3800. [Google Scholar] [CrossRef] [PubMed]

| Ink Used | [Glycerine] (v/v) | Cure Temperature | Cure Time | Number of Ink Layers | Resulting Electrodes |
|---|---|---|---|---|---|
| AgNW aqueous ink | 1% | 60 °C 1 | 10 min | 1 |  |
| 2% |  | ||||
| AgNW 0.5% (w/w) suspension | 2% | 75 °C | 30 min | 2 |  |
| 3 |  | ||||
| Chi-LaA-AgNW dispersion, Chi:AgNW (1:1) | 1% | 75 °C 2 | 30 min | 1 |  |
| 2 |  | ||||
| 3 |  | ||||
| 2% | 15 min | 2 |  | ||
| 30 min | 1 |  | |||
| 2 |  | ||||
| 3 |  | ||||
| 4 |  | ||||
| 1 h | 2 |  | |||
| 3 |  | ||||
| 2 h | 2 |  | |||
| Chi-LaA-AgNW dispersion, Chi:AgNW (2:1) | 2% | 75 °C | 30 min | 2 |  |
| 3 |  | ||||
| 1 h | 2 |  | |||
| 3 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.L.; Martins, G.V. Biocompatible and Flexible Transparent Electrodes for Skin-Inspired Sensing. Eng. Proc. 2023, 35, 26. https://doi.org/10.3390/IECB2023-14588
Pereira RL, Martins GV. Biocompatible and Flexible Transparent Electrodes for Skin-Inspired Sensing. Engineering Proceedings. 2023; 35(1):26. https://doi.org/10.3390/IECB2023-14588
Chicago/Turabian StylePereira, Raquel L., and Gabriela V. Martins. 2023. "Biocompatible and Flexible Transparent Electrodes for Skin-Inspired Sensing" Engineering Proceedings 35, no. 1: 26. https://doi.org/10.3390/IECB2023-14588
APA StylePereira, R. L., & Martins, G. V. (2023). Biocompatible and Flexible Transparent Electrodes for Skin-Inspired Sensing. Engineering Proceedings, 35(1), 26. https://doi.org/10.3390/IECB2023-14588







