Ag/TiO2 Nanocomposites for Nanothermometry in the Biological Environment †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag NPs
2.3. Synthesis of Ag@TiO2
2.4. Synthesis of TiO2 NPs
2.5. Optical Characterization
3. Results and Discussion
3.1. Characterization
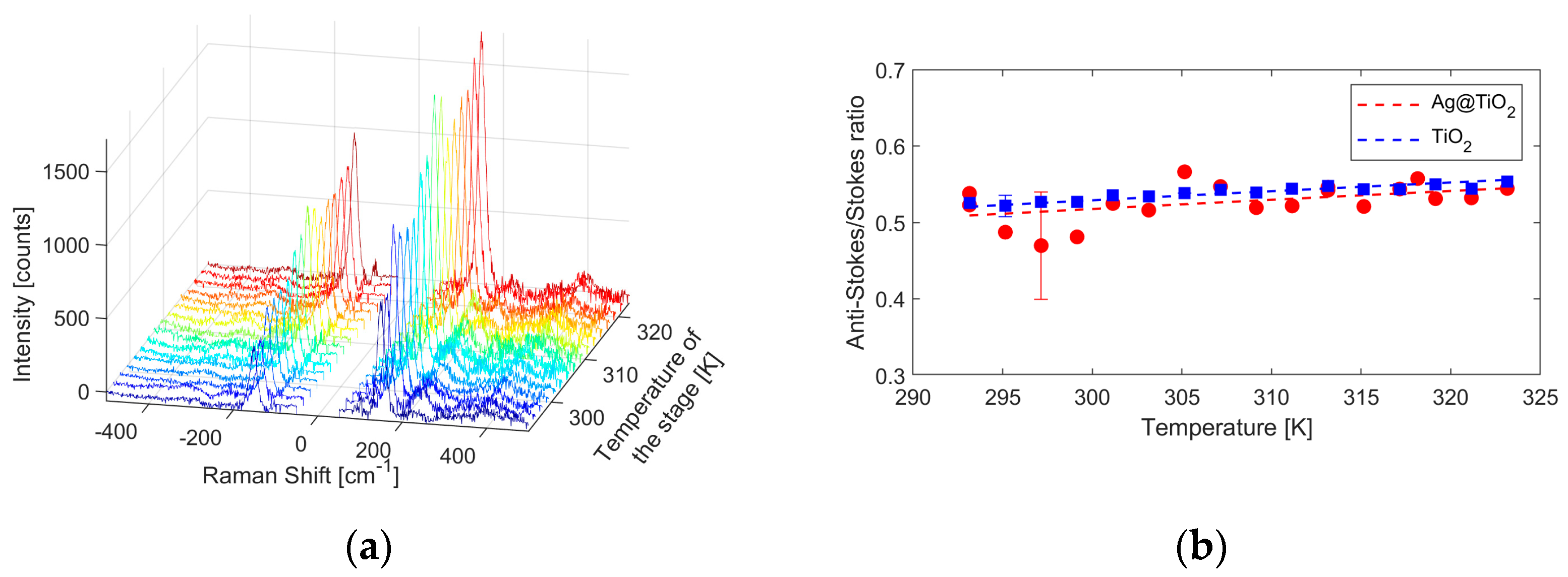
3.2. Raman Nanothermometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradac, C.; Lim, S.F.; Chang, H.-C.; Aharonovich, I. Optical Nanoscale Thermometry: From Fundamental Mechanisms to Emerging Practical Applications. Opt. Mater. 2020, 8, 2000183. [Google Scholar] [CrossRef]
- Quintanilla, M.; Liz-Marzán, L. Guiding Rules for Selecting a Nanothermometer. Nano Today 2018, 19, 126–145. [Google Scholar] [CrossRef]
- Piñol, R.; Brites, C.D.S.; Silva, N.J.; Carlos, L.D.; Millán, A. Chapter 6 - Nanoscale Thermometry for Hyperthermia Applications. In Micro and Nano Technologies. In Nanomaterials for Magnetic and Optical Hyperthermia Applications, 1st ed.; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–172. [Google Scholar] [CrossRef]
- Gao, P.; Wang, H.; Cheng, Y. Strategies for efficient photothermal therapy at mild temperatures: Progresses and challenges. Chin. Chem. Lett. 2022, 33, 575–586. [Google Scholar] [CrossRef]
- Zani, V.; Pedron, D.; Pilot, R.; Signorini, R. Contactless Temperature Sensing at the Microscale Based on Titanium Dioxide Raman Thermometry. Biosensors 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844. [Google Scholar] [CrossRef] [PubMed]
- Pretto, T.; Franca, M.; Zani, V.; Gross, S.; Pedron, D.; Pilot, R.; Signorini, R. A Sol-Gel/Solvothermal Synthetic Approach to Titania Nanoparticles for Raman Thermometry. Sensors 2023, 23, 2596. [Google Scholar] [CrossRef] [PubMed]
- Yguerabide, J.; Yguerabide, E.E. Light-Scattering Submicroscopic Particles as Highly Fluorescent Analogs and Their Use as Tracer Labels in Clinical and Biological Applications: I. Theory. Anal. Biochem. 1998, 262, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Koktysh, D.; Mamedov, A.; Giersig, M.; Kotov, N.; Liz-Marzán, L. One-Pot Synthesis of Ag@TiO2 Core-Shell Nanoparticles and Their Layer-by-Layer Assembly. Langmuir 2000, 16, 2731–2735. [Google Scholar] [CrossRef]
- Wang, P.; Wang, D.; Xie, T.; Li, H.; Yang, M.; Wei, X. Preparation of monodisperse Ag/Anatase TiO2 core–shell nanoparticles. Mater. Chem. Phys. 2008, 109, 108–183. [Google Scholar] [CrossRef]
- Demirörs, A.; van Blaaderen, A.; Imhof, A. A General Method to Coat Colloidal Particles with Titania. Langmuir 2010, 26, 9297–9303. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Lyu, L.M.; Koga, K.; Shimoyama, Y.; Kon, Y. Plasmonic Ag@TiO2 Core−Shell Nanoparticles for Enhanced CO2 Photoconversion to CH4. ACS Sustain. Chem. Eng. 2019, 7, 18955–18964. [Google Scholar] [CrossRef]
- Karimipour, M.; Ebrahimi, M.; Abafat, Z.; Molaei, M. Synthesis of Ag@TiO2 core-shells using a rapid microwave irradiation and study of their nonlinear optical properties. Opt. Mater. 2016, 57, 257–263. [Google Scholar] [CrossRef]
- Zhang, D.; Song, X.; Zhang, R.; Zhang, M.; Liu, F. Preparation and Characterization of Ag@TiO2 Core-Shell Nanoparticles in Water-in-Oil Emulsions. EurJIC 2005, 9, 1643–1648. [Google Scholar] [CrossRef]
- Lee, P.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Lee, W.; Yeop, J.; Heo, J.; Yoon, Y.J.; Park, S.; Jeong, J.; Shin, Y.; Kim, J.; An, N.; Kim, D.; et al. High colloidal stability ZnO nanoparticles independent on solvent polarity and their application in polymer solar cells. Sci. Rep. 2020, 10, 18055. [Google Scholar] [CrossRef] [PubMed]
- Giarola, M.; Sanson, A.; Monti, F.; Mariotto, G.; Bettinelli, M.; Speghini, A.; Salviulo, G. Vibrational Dynamics of Anatase TiO2: Polarized Raman Spectroscopy and Ab Initio Calculations. Phys. Rev. B 2010, 81, 174305-1–174305-7. [Google Scholar] [CrossRef]
- Joshi, N.; Jain, N.; Pathak, A.; Singh, J.; Prasad, R.; Upadhyaya, C.P. Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J. Sol-Gel Sci. Technol. 2018, 86, 682–689. [Google Scholar] [CrossRef]

| Sample | Laser Power [mW] | Experimental Constant | Sensitivity at 295.15 K [K−1] | Relative Sensitivity [K−1%] |
|---|---|---|---|---|
| Ag@TiO2 | 1.44 | 1.007 ± 0.020 | 0.0012 | 0.26 |
| TiO2 | 1.52 | 1.007 ± 0.004 | 0.0012 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambon, R.; Franca, M.; Zani, V.; Pilot, R.; Gross, S.; Pedron, D.; Signorini, R. Ag/TiO2 Nanocomposites for Nanothermometry in the Biological Environment. Eng. Proc. 2023, 35, 16. https://doi.org/10.3390/IECB2023-14585
Zambon R, Franca M, Zani V, Pilot R, Gross S, Pedron D, Signorini R. Ag/TiO2 Nanocomposites for Nanothermometry in the Biological Environment. Engineering Proceedings. 2023; 35(1):16. https://doi.org/10.3390/IECB2023-14585
Chicago/Turabian StyleZambon, Roberto, Marina Franca, Veronica Zani, Roberto Pilot, Silvia Gross, Danilo Pedron, and Raffaella Signorini. 2023. "Ag/TiO2 Nanocomposites for Nanothermometry in the Biological Environment" Engineering Proceedings 35, no. 1: 16. https://doi.org/10.3390/IECB2023-14585
APA StyleZambon, R., Franca, M., Zani, V., Pilot, R., Gross, S., Pedron, D., & Signorini, R. (2023). Ag/TiO2 Nanocomposites for Nanothermometry in the Biological Environment. Engineering Proceedings, 35(1), 16. https://doi.org/10.3390/IECB2023-14585










