Nanoparticle/DNAzyme Based Biosensors for Heavy-Metal Ion Detection: Effect of DNAzyme Surface Modifications on Device Sensitivity †
Abstract
:1. Introduction
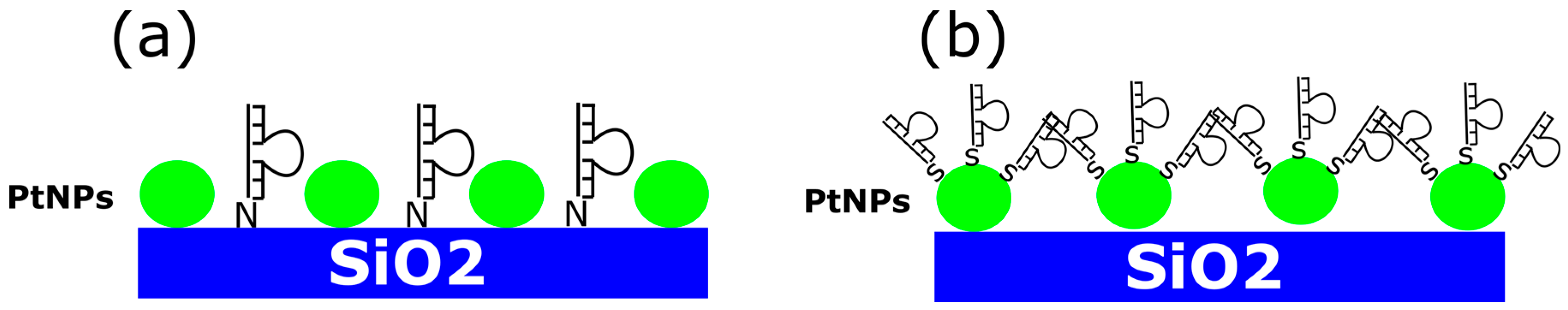
2. Materials and Methods
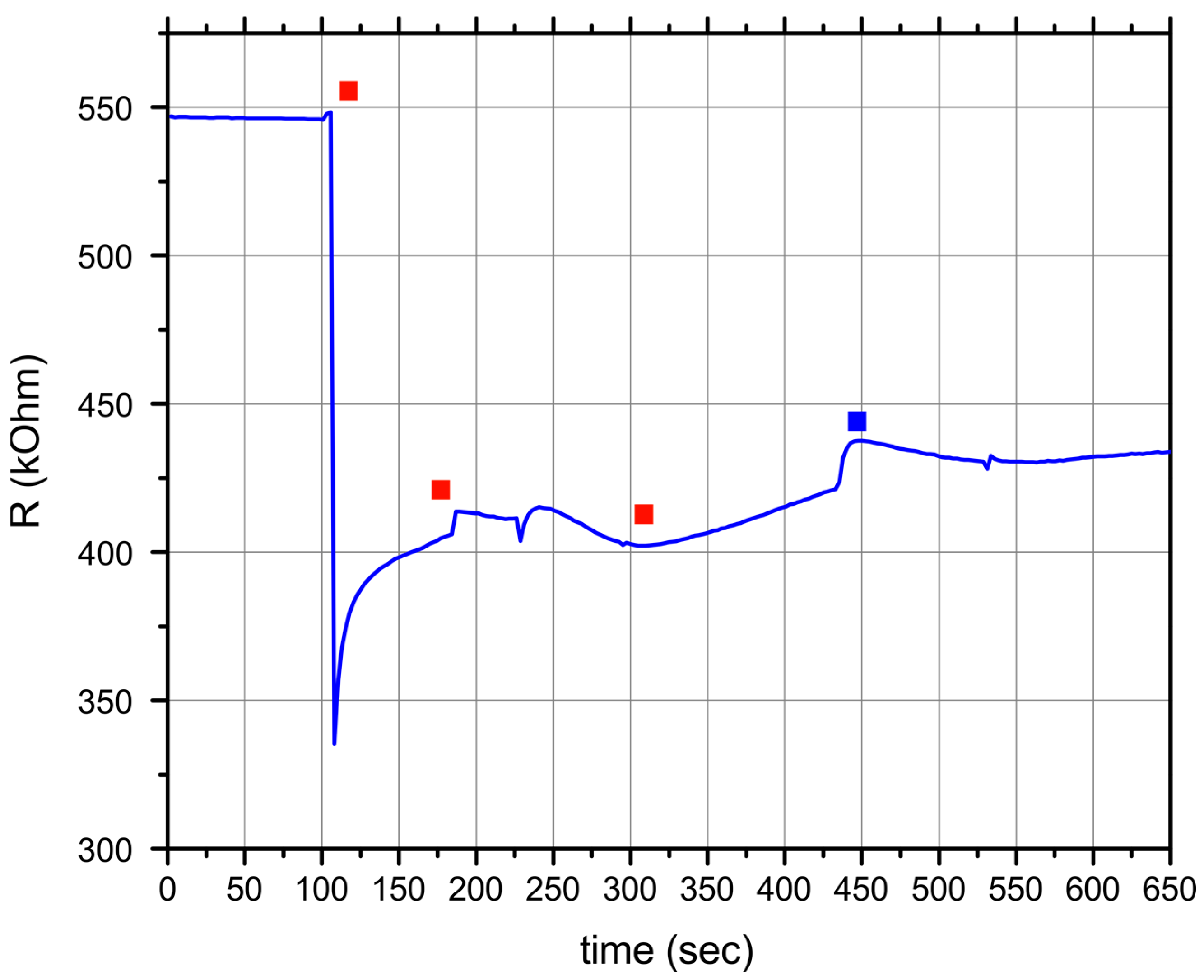
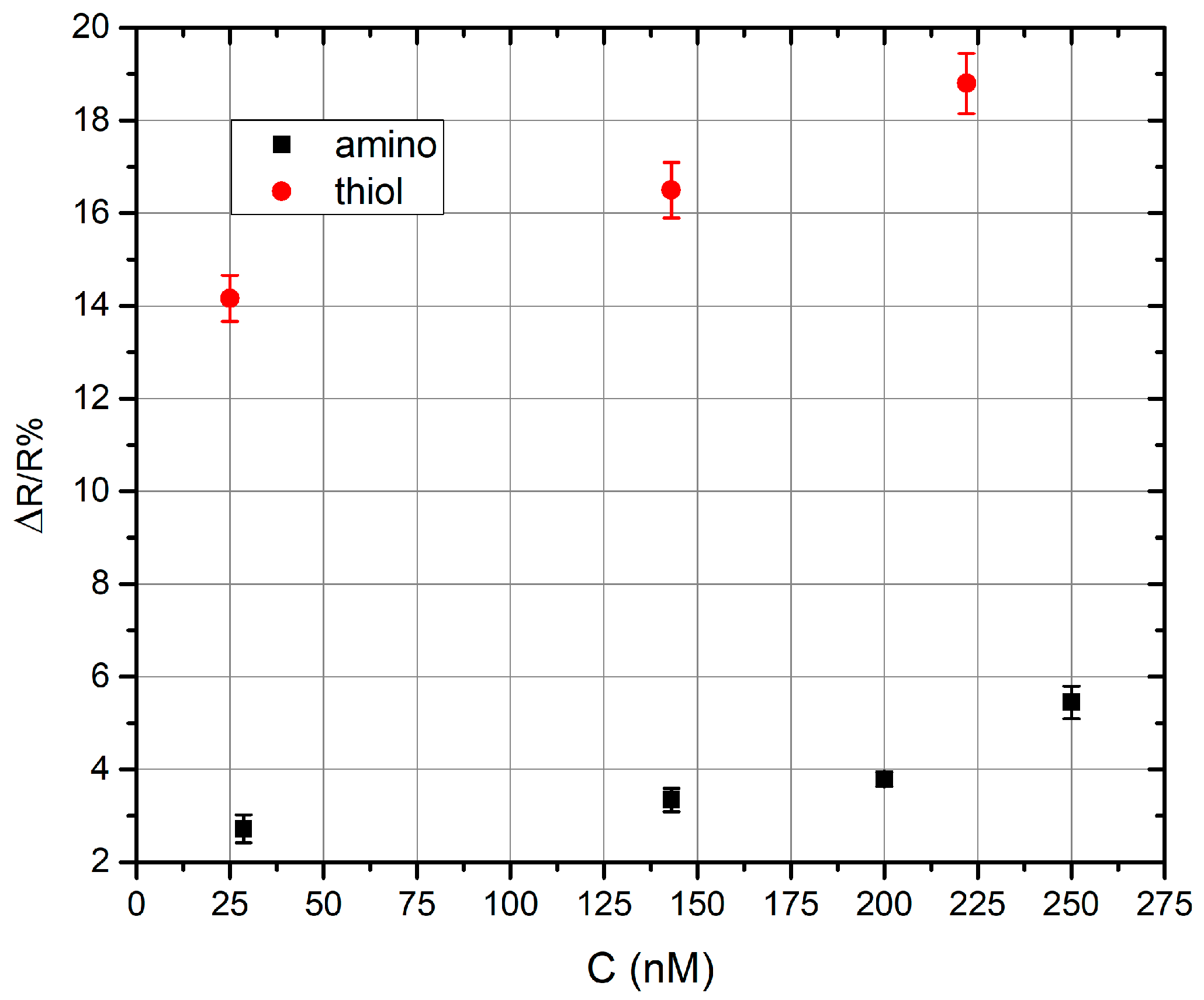
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Yavari, K.; Wang, Y.; Van Cappellen, P.; Liu, J. Deployment of functional DNA-based biosensors for environmental water analysis. Trends Anal. Chem. 2022, 153, 116639. [Google Scholar]
- Skotadis, E.; Tsekenis, G.; Chatzipetrou, M.; Zergioti, I.; Tsoukalas, D. Heavy metal ion detection using DNAzyme-modified platinum nanoparticle networks. Sens. Actuators B Chem. 2017, 239, 962–969. [Google Scholar]
- Skotadis, E.; Voutyras, K.; Chatzipetrou, M.; Tsekenis, G.; Patsiouras, L.; Madianos, L.; Chatzandroulis, S.; Ioanna, Z.; Tsoukalas, D. Label-free DNA biosensor based on resistance change of platinum nanoparticles assemblies. Biosens. Bioelectron. 2016, 81, 388–394. [Google Scholar]
- European Parliament, Council of the European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020, on the quality of water intended for human consumption. Off. J. Eur. Union 2020, 435, 1–62. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslanidis, E.; Skotadis, E.; Panagopoulou, C.; Rapesi, A.; Tzourmana, G.; Tsekenis, G.; Tsoukalas, D. Nanoparticle/DNAzyme Based Biosensors for Heavy-Metal Ion Detection: Effect of DNAzyme Surface Modifications on Device Sensitivity. Eng. Proc. 2023, 35, 17. https://doi.org/10.3390/IECB2023-14581
Aslanidis E, Skotadis E, Panagopoulou C, Rapesi A, Tzourmana G, Tsekenis G, Tsoukalas D. Nanoparticle/DNAzyme Based Biosensors for Heavy-Metal Ion Detection: Effect of DNAzyme Surface Modifications on Device Sensitivity. Engineering Proceedings. 2023; 35(1):17. https://doi.org/10.3390/IECB2023-14581
Chicago/Turabian StyleAslanidis, Evangelos, Evengelos Skotadis, Chryssi Panagopoulou, Annita Rapesi, Georgia Tzourmana, George Tsekenis, and Dimitris Tsoukalas. 2023. "Nanoparticle/DNAzyme Based Biosensors for Heavy-Metal Ion Detection: Effect of DNAzyme Surface Modifications on Device Sensitivity" Engineering Proceedings 35, no. 1: 17. https://doi.org/10.3390/IECB2023-14581
APA StyleAslanidis, E., Skotadis, E., Panagopoulou, C., Rapesi, A., Tzourmana, G., Tsekenis, G., & Tsoukalas, D. (2023). Nanoparticle/DNAzyme Based Biosensors for Heavy-Metal Ion Detection: Effect of DNAzyme Surface Modifications on Device Sensitivity. Engineering Proceedings, 35(1), 17. https://doi.org/10.3390/IECB2023-14581








