Abstract
In this study, a graphene oxide-modified screen-printed carbon electrode was functionalized with the 1-pyrenebutyric acid-N-hydroxy-succinimide ester and conjugated with antibodies for the label-free detection of human hepatoma HepG2 cells. Using a user-friendly reservoir chamber, the functionalized film was exposed continuously to the cancer cells. The use of a continuous flow was intended to enhance the capture of the target cells by the sensing platform. The response of the biosensor was evaluated using cyclic voltammetry. The preliminary data showed good sensitivity for the detection of hepatic cancer cells. The developed biosensor could detect the HepG2 cells from a 1 × 103 to 3 × 105 cells/mL range. Thus, it is a simple tool for the electrochemical detection of cancer cells and offers a low-cost and disposable sensing platform.
1. Introduction
Electrochemical biosensors are popular tools in the field of analytical chemistry due to their high sensitivity and selectivity for the detection of analytes of interest. These biosensors are highly effective for different applications such as the medical, pharmaceutical, environmental, and food sectors because they use the principles of electrochemistry to convert biological interactions into quantifiable signals [1]. Cyclic voltammetry (CV) is one of the most commonly used techniques for electrochemical biosensors. CV measures the current and voltage via reduction and oxidation reactions by applying a voltage sweep to a working electrode surface and monitoring the current response as a function of potential. The resulting signal provides information about the electrochemical behavior of the sensor, including the redox potential, electron transfer kinetics, and the concentration of different analytes such as small molecules, proteins, and nucleic acids [1,2]. Cyclic voltammetry is highly versatile and can be adapted to a variety of electrode materials, including metals, carbon, and conducting polymers. The sensitivity and selectivity of a fabricated biosensor can be enhanced using electrode modifications, such as the addition of nanoparticles, linkers, antibodies, enzymes or other biomolecules such as surface-layer proteins [3,4,5].
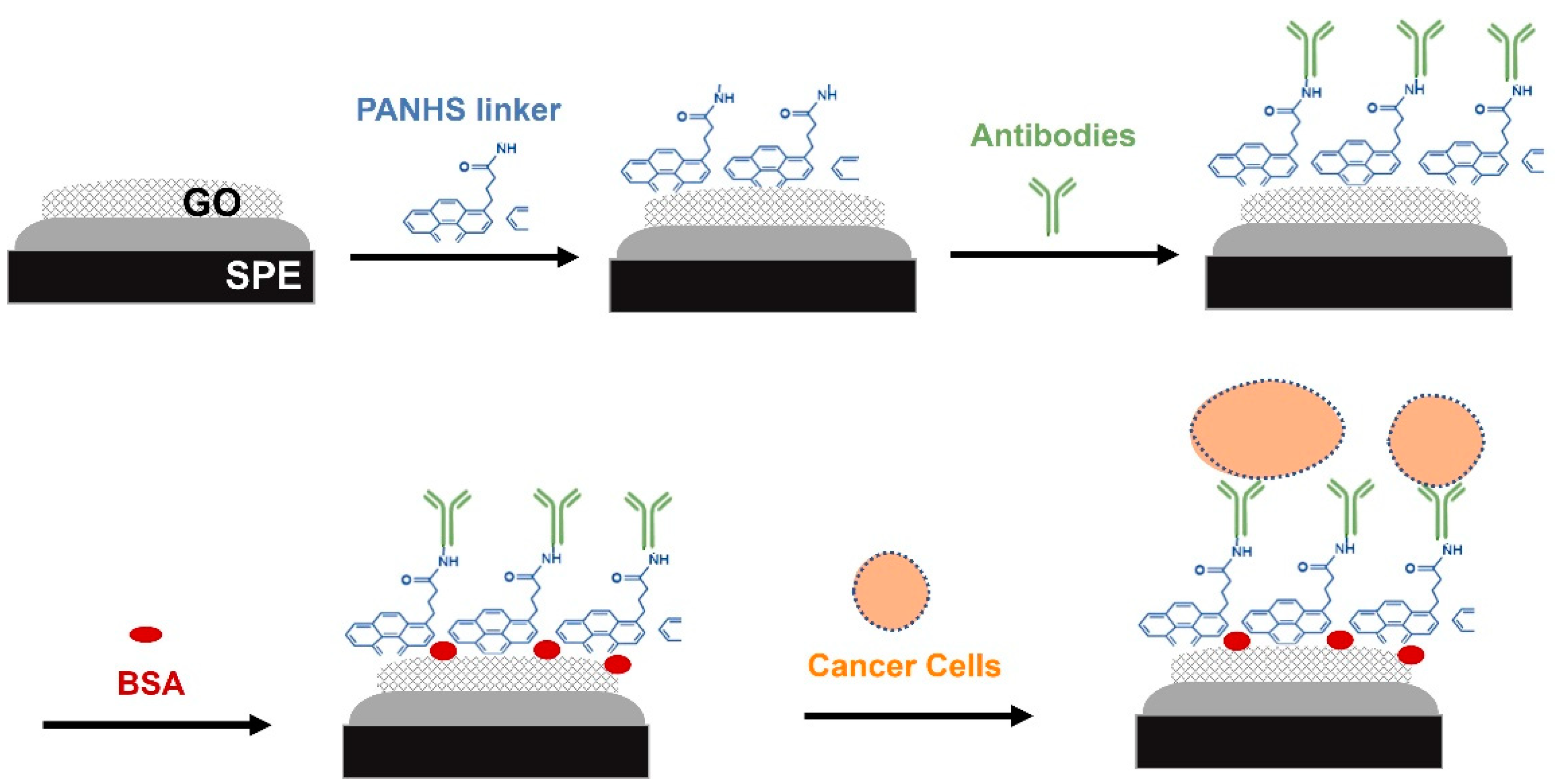
Graphene has seen increasing attention in recent years. It is a 2D sheet of carbon atoms that are arranged hexagonally and exposed on its surface [2,6]. Graphene, graphene oxide (GO) and reduced graphene oxide (rGO) are biocompatible carbon-based materials that are lately used in bioelectrochemical systems. These materials have been employed in many biosensing platforms due to their chemical stability, high electronic conductivity, large specific area, and high carrier mobility [7]. GO is synthesized by chemically oxidizing graphite and then can be immobilized on a solid support and functionalized with capturing molecules such as antibodies and a nucleic acid probe [8]. To improve the selectivity and sensitivity of fabricated biosensors, the immobilization of antibodies in the correct orientation on the electrode surface is critical step [5,8]. Therefore, linker molecules can be used as scaffolding molecules that offer an appropriate base for immobilizing antibodies [9]. In this study, the 1-pyrenebutyric acid-N-hydroxy-succinimide ester (PANHS) was immobilized on a GO electrode through π stacking, while the succinimidyl ester group was bound to the amine groups of an antibody. In the proposed label-free electrochemical-based biosensor, screen-printed carbon electrodes modified with GO were functionalized with the scaffolding molecule, PANHS, and capturing molecule, the anti-OV-6 antibody to detect intact hepatic cancer cells (HepG2) (Figure 1). Further, a reservoir chamber module was used to enable the continuous exposure of cancer cells to the functionalized surface and thus increase the capture of target cells in the specimen. By optimizing the detection conditions and assessing the generated signals from CV measurements, promising biosensing platforms were developed.
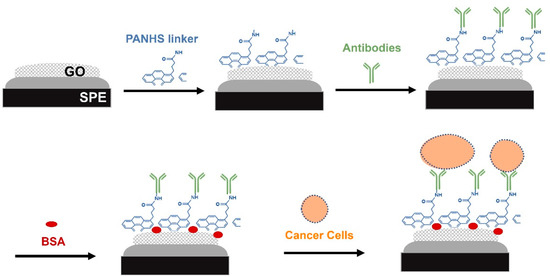
Figure 1.
Schematic representation of the developed electrochemical biosensor for detection of cancer cells in the HepG2 cell line.
2. Materials and Methods
2.1. Fabrication of the Sensing Platform
A three-electrode system (Zimmer & Peacock Ltd., UK) consisting of a counter electrode (CE, carbon), reference electrode (RE, Ag/AgCl), and working electrode (WE; the diameter of carbon electrodes is 4 mm, and they are functionalized with GO) was used to perform the electrochemical measurements. Initially, 2 mM PANHS (Sigma Aldrich, UK) in methanol was applied to cover the surface of the GO electrode. After incubation for at least 4 h at 4 °C, 50 μg/mL of the anti-OV-6 antibody (R&D Systems, UK) in PBS was dropped onto the modified surface and incubated at 4 °C overnight, followed by blocking with 1% BSA in PBS for 1 h to minimize unspecific adsorption on the activated surface. A rinsing step with PBS was performed after each step.
2.2. Electrochemical Measurements
CV measurements for each functionalized layer of the fabricated sensor and after cell injection in a solution of 5 mM [Fe(CN6)]3− and 5 mM [Fe(CN6)]4− (1:1) containing 100 mM KCl and cyclic voltammograms were recorded from −0.6 to 0.6 V at scan rate of 0.05 V/s. Indeed, the developed electrodes were embedded into a reservoir chamber module that allowed HepG2 cell injection at different numbers and continuous exposure to the developed sensor.
2.3. Cell Lines and Cell Culture
The human liver hepatocellular carcinoma cell line (HepG2) (American Type Culture Collection) was cultured in DMEM supplemented with 10% fetal bovine serum (FBS), and a 1% antibiotic/antimycotic solution at 37 °C in a 5% CO2 and 95% air humidified atmosphere. After 48 h, the cells were detached using trypsin and separated from the medium via centrifugation.
3. Results and Discussion
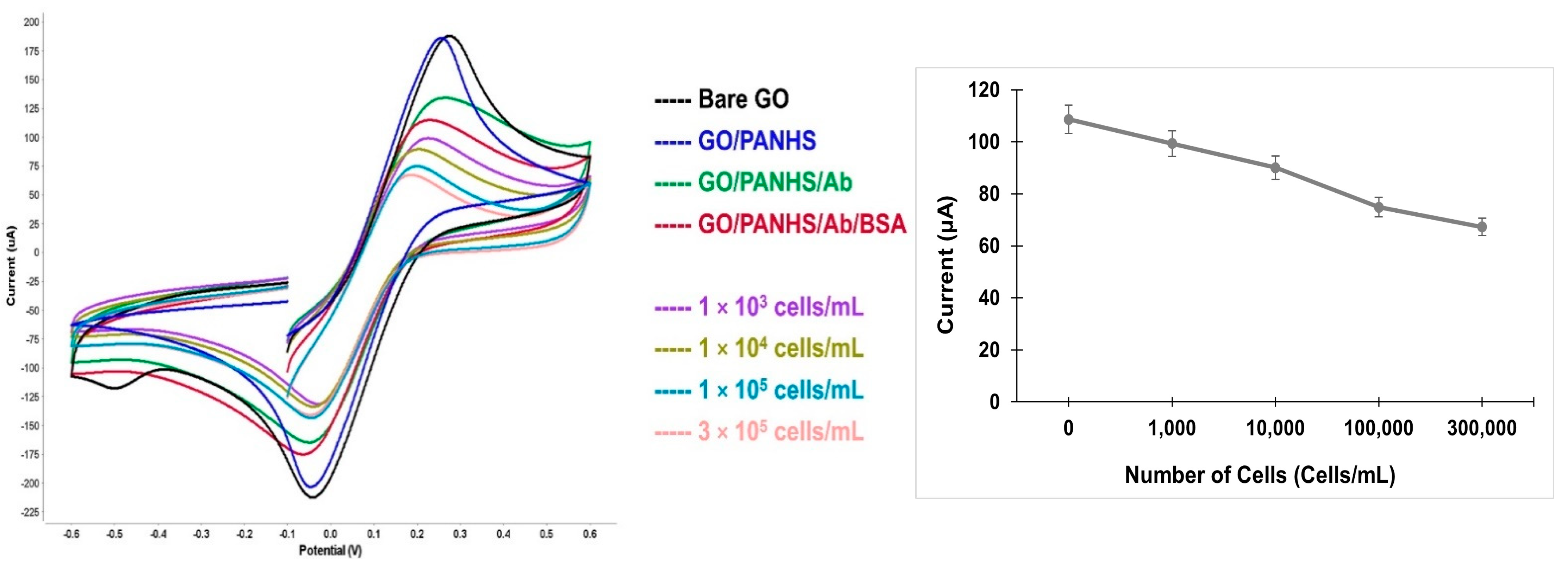
This study aimed to design a simple approach to detect intact cells by using an electrochemical technique. The fabricated electrochemical sensor was characterized using CV measurements and all measurements were conducted in a 5 mM ([Fe(CN)6]3−/4−) redox couple and 100 mM KCl. CV is a powerful electrochemical method that is commonly used to monitor the changes in modified sensor surfaces. To confirm the functionalization of the developed sensor on the GO electrode, CV measurements were taken after each electrode modification step as follows: bare GO, GO/PANHS, GO/PANHS/Ab, and GO/PANHS/Ab/BSA electrodes. The current response decreased noticeably after each addition step taken on the electrode surface (Figure 2).
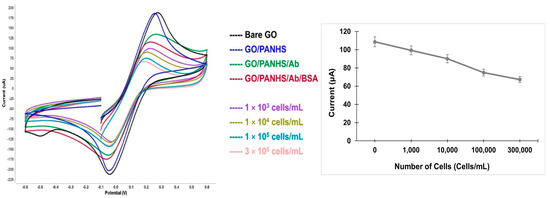
Figure 2.
Cyclic voltammetry spectra of the developed GO/PANHS/Ab/BSA sensor with different numbers of hepatic cancer cells (left). Peak current values as a function of the number of cells that were added onto the developed sensor (right).
The bare GO surface showed the highest peak current due to a small resistance in the electron transfer. The noticeable change in the peak current for GO/PANHS compared to that in the bare GO electrode shows the formation of an additional layer. The further reduction in the CV current peaks for GO/PANHS/Ab and GO/PANHS/Ab/BSA indicate the immobilization of the antibodies and the steric hindrance of BSA molecules in the electron transfer, respectively, which attributed to the decrease in electron transportation during oxidation and reduction reactions. These changes in the electron transfer rate reflect the successful fabrication of the sensing platform. Subsequently, a suspension of HepG2 cells was added to the fabricated sensor at different cell densities, and CV measurements were conducted in the presence of cancer cells. The ability of the developed biosensor to capture cancer cells due to the specific antigen–antibody interaction was verified by the CV spectra. When the cell suspension was added to the functionalized electrode, the peak current decreased relative to the initial value measured in the absence of cells. The cyclic voltammograms of the blank test (no cells) and those with cells are presented in Figure 2. The peak current consistently decreased as the number of captured cells increased. The HepG2 cancer cell line can be identified by several cell surface markers such as OV6. Further, using the chamber reservoir offers a continuous-flow system that improves the capturing efficiency by allowing the continuous exposure of the cells to the functionalized sensor. Cell injection resulted in the generation of well-defined cyclic voltammograms at different cell numbers. The voltammograms show peak shifts corresponding to increasing cell numbers because captured cells blocked the direct access of the ions to the electrode surface.
4. Conclusions
This work offers a simple platform for diagnostic purposes that enables the use of disposable screen-printed electrodes and a chamber reservoir for the continuous exposure of cancer cells to the fabricated electrode. The developed GO-modified electrochemical sensor was efficient in capturing hepatic cells via recognition of the OV6 marker that was highly expressed on the cancer cell surface. The obtained results present the viability of this potentially low-cost, effective, and reliable biosensor for cell detection. This electrochemical platform could be further modified with other biomarkers to detect other types of cancer and may be useful for point-of-care diagnostics.
Author Contributions
Conceptualization, S.D., S.A.A., M.P. and B.S.; methodology, S.D.; formal analysis, S.D.; investigation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, S.A.A., M.P. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damiati, S.; Schuster, B. Electrochemical biosensors based on S-layer proteins. Sensors 2020, 20, 1721. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.; Banks, C. Interpreting Electrochemistry. In The Handbook of Graphene Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 23–77. [Google Scholar]
- Damiati, S.; Peacock, M.; Mhana, R.; Søpstad, S.; Sleytr, U.B.; Schuster, B. Bioinspired Detection Sensor Based on Functional Nanostructures of S-Proteins to Target the Folate Receptors in Breast Cancer Cells. Sens. Actuators B 2018, 267, 224–230. [Google Scholar] [CrossRef]
- Bungon, T.; Haslam, C.; Damiati, S.; O’Driscoll, B.; Whitley, T.; Davey, P.; Siligardi, G.; Charmet, J.; Awan, S.A. Graphene FET sensors for Alzheimer’s disease protein biomarker Clusterin detection. Front. Mol. Biosci. 2021, 8, 651232. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B. S-Layer Protein-Based Biosensors. Biosensors 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E. Experimental review of graphene. Int. Scholarly Res. Notices 2012, 2012, 501686. [Google Scholar] [CrossRef]
- Damiati, S.; Haslam, C.; Sopstad, S.; Peacock, M.; Whitley, T.; Davey, P.; Awan, S.A. Sensitivity comparison of macro- and micro-electrochemical biosensors for human chorionic gonadotropin (hCG) biomarker detection. IEEE Access 2019, 7, 94048–94058. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Haslam, C.; Damiati, S.; Whitley, T.; Davey, P.; Ifeachor, E.; Awan, S.A. Label-free sensors based on graphene field-effect transistors for the detection of human chorionic gonadotropin cancer risk biomarker. Diagnostics 2018, 8, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).