Abstract
Milk is a widely consumed product, and its adulteration is not only common but also very dangerous. This study aimed to develop a biosensor for the detection of milk adulteration using DNA hybridization coupled with an electrochemical device. The advantages of biosensors over traditional laboratory methods, such as their speed, ease of use, and cost-effectiveness, are combined with the sensitivity of DNA hybridization. A capacitive biosensor was developed using interdigitated gold electrodes on paper substrate, which were modified with specific oligonucleotides for cow mitochondrial DNA that served as the biorecognition element. The methodology relies on the measurement of changes in capacitance due to DNA hybridization. Preliminary results are presented, showing the ability of this biosensor to detect bovine DNA in goat milk with high sensitivity and specificity. The results show that this biosensor has the potential to be a low-cost, easy-to-perform, and fast method for the detection of milk adulteration. This biosensor technology is a promising development for the detection of milk adulteration and can help to ensure the safety and quality of milk products.
1. Introduction
Milk is a vital source of nutrition for many people around the world, and its purity and quality are of great importance for human health. However, milk adulteration is a serious and yet common problem. Some of the typical milk adulterants such as water reduce its nutrient value [1]. Others, such as milk from different sources, are used mainly for financial reasons and are really hard to detect [2], while others such as commercial urea, melamine, or plant proteins falsely improve quality parameters of the milk [3] but can also lead to allergies or serious and long-term health risks [4].
In recent years, there has been an increase in awareness of the milk adulteration problem, and various methods have been developed to detect it [5]. However, many of these methods are time-consuming, complicated, and expensive; therefore, there is a need for a more efficient and cost-effective method for the detection of milk adulteration.
Biosensors are a promising technology for the detection of milk adulteration, as they offer several advantages over traditional laboratory methods [6]. They are based on the principle of biological recognition, which allows them to detect specific molecules with high sensitivity and specificity. DNA hybridization is a powerful technique that can be used in biosensors, as it is highly specific and can detect even small amounts of DNA.
Interdigitated gold electrode capacitors (IDGECs) are a type of biosensor that have been used for the detection of various analytes, including DNA [7]. The main concept according to which these biosensors function is the change of the capacitance in the different stages of the process of their development. The basic equation according to which the IDE capacitance changes is given in Equation (1) [8]:
According to Equation (1), capacitance (C) is dependent on the surface area of the electrodes (A), the vacuum permittivity (ε0), the relative permittivity of the medium between the plates (εr), and the distance (d) between the electrodes [9].
This study aims to develop a biosensor for the detection of milk adulteration combining DNA hybridization and the properties of IDGECs, as well as to evaluate the biosensor’s potential as a low-cost, easy-to-perform, and fast method. The significance of this study is that it contributes to the development of a more efficient and cost-effective method for the detection of milk adulteration, which can help to ensure the safety and quality of milk products and protect human health.
2. Materials and Methods
For the detection of bovine DNA as adulteration in dairy products of other sources (e.g., goat milk), a capacitive DNA-hybridization-based biosensor with gold interdigitated electrodes was developed.
2.1. Structure of the Biosensor
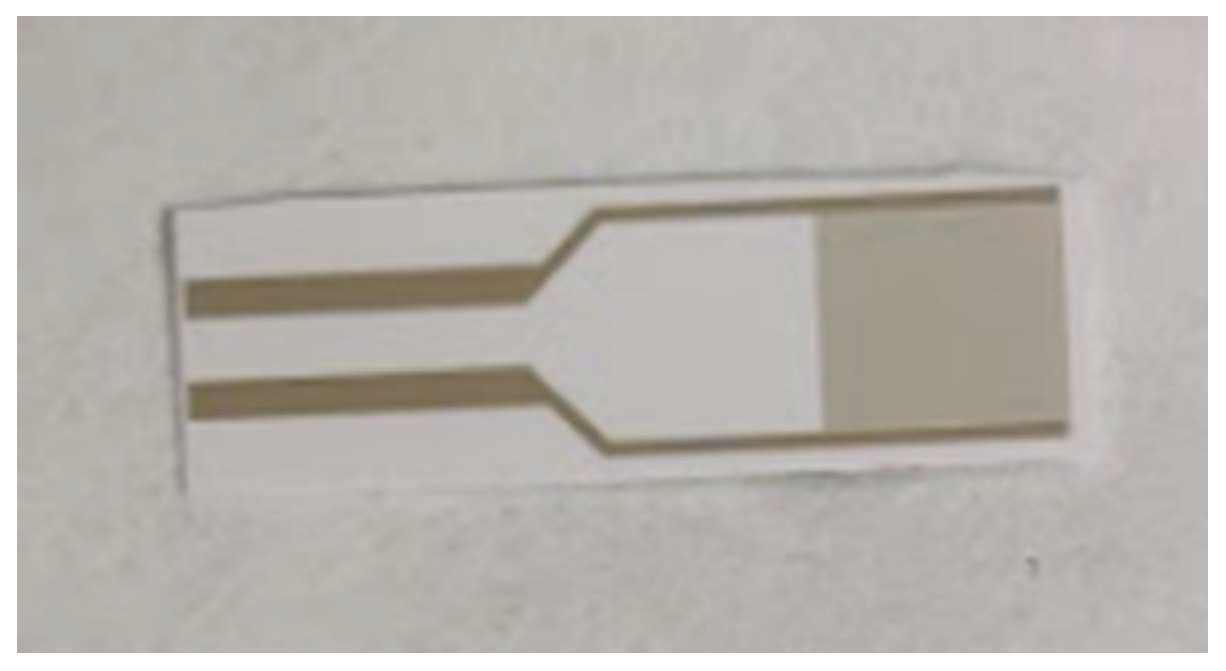
The gold interdigitated capacitor of Figure 1 was used as the transducer of the biosensor. It was purchased from DropSens (Asturias, Spain), cat. N.: PW-IDEAU50 and each element contains 70 gold electrodes with 50 μm width each, 7 mm total length and 8.45 mm2 total surface area. Two specific oligonucleotides for cow mitochondrial DNA were used as biorecognition elements: forward CAATAACTCAACACAGAATTT (COWFORW) and reverse CGTGATCTAATGGTAAGGAAT (COWREV). Each primer was modified in the 5′-end with a thiol group. An original screening device that was developed in the laboratory was used in order to detect changes in the capacitance [10]. The device was connected to a computer and the data collected were saved there according to the defined settings.
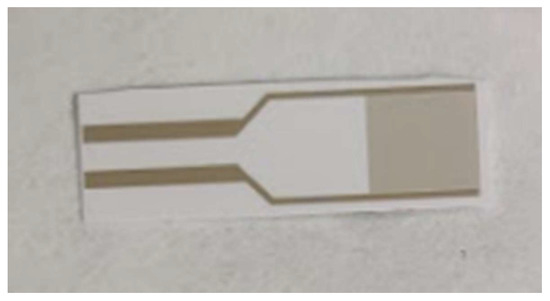
Figure 1.
Interdigitated gold electrode capacitor.
2.2. Development of Biosensor
2.2.1. Selection and Preparation of Samples
The samples of the DNA were provided by the laboratory of the University of West Attica and were extracted as described in [11]. After the purchase of different milk products (samples) of different brands from local supermarkets in Athens, the DNA had to be extracted. DNA was extracted from 1.5 mL of each sample after centrifugation (10 min at 12,000× g). The NucleoSpin Food® kit (Macherey-Nagel GmbH & Co. KG, Dueren, Germany) was used, following the manufacturer’s instructions, with a slight modification of incubating the sample overnight at 65 °C with the Lysis Buffer and Proteinase K, instead of the recommended 30 min incubation. Quantification of the extracted DNA was performed using a spectrophotometer at a wavelength of 260 nm [11].
2.2.2. Prepatation of Primers
The primers were purchased as dried pellets and therefore were resuspended according to the manufacturer’s instructions. Primers’ concentration was 100 pmol/μL. The stock solutions were prepared through adding the appropriate amount of molecular-biology-grade water, divided into aliquots of 60 μL and stored in −20 °C until use.
2.2.3. Preparation of Interdigitated Capacitors
In order to obtain a detectable hybridization between primers and the target DNA, primers must be immobilized at the capacitors’ surface. The immobilization process is based on the affinity reaction between the sulfate (thiol) group of the primers and the gold surface of the electrodes, during which a covalent bond is formed between Au and S. That covalent bond leads to an increase in the capacitance of the IDE capacitor [12].
In order to measure the changes, an initial measurement was taken before the immobilization process, after adding 30 μL of PBS (phosphate-buffered saline) at the surface of the capacitors.
For the immobilization, mixtures that contained 2 mL of PBS and 50 μL of primer were used. The primer was either the forward (COWFORW) or the reverse (COWREV) or 25 μL of each one in order to test whether the presence of both might increase sensitivity. For each mixture, two capacitors were placed with the gold surface always in contact with the solution and were stored overnight (15–20 h) at 4 °C. After that they were washed for 2–3 min in PBS to remove the unattached primers, then they were dried and finally their capacitance was measured with 30 μL of PBS at the surface in order to see the change caused by the immobilization. The experiments were carried out with 36 capacitors—12 for each different primer mixture.
2.2.4. Negative Control
Negative control tests were performed using non-complementary goat DNA and with molecular biology water, which was the solvent for primers and all others DNA samples.
For the non-complementary DNA test, a sample of goat DNA extracted from commercially available milk was used at a concentration 1 ng/μL. After the immobilization of the primers and the washing steps, 30 μL of goat DNA mixture was placed on the surface of three interdigitated capacitors. Each interdigitated capacitor was prepared with either one of or both primers. The capacitance was measured with the measuring device every second for 4 min and 30 s.
For the molecular biology water test, 30 μL of molecular biology water was placed at the surface of three capacitors; each of them was prepared with either one or both of primers. The capacitance was measured with the measuring device every second for 2 min. The time interval was smaller because the capacitance, in this case, stabilized faster compared to the first non- complementary goat DNA test.
2.2.5. DNA Detection
In order to measure the changes in capacitance caused by the presence of bovine DNA, a sample of DNA extracted from cow milk was used. Its concentration was measured with the spectrophotometer and using molecular biology grade water a solution with concentration of 1.333 ng/μL was prepared. After the immobilization of the primers and the washing process, 30 μL of the cow DNA mixture was placed at the surface of 12 interdigitated capacitors (4 prepared only with COWFORW primer, 4 prepared only with COWREV primer and 4 prepared with both COWFORW and COWREV primers) and the capacitance was measured every second for 4 min and 30 s.
3. Results and Discussion
The developed biosensor was based on the hybridization between DNA primers that are immobilized on the gold interdigitated surface of the capacitors and the target DNA. Detection of hybridization indicates that the dairy product contains cow DNA. If such hybridization is detected in dairy products that are not derived from bovines, the adulteration is confirmed. Preliminary results of the development of this biosensor are presented herein.
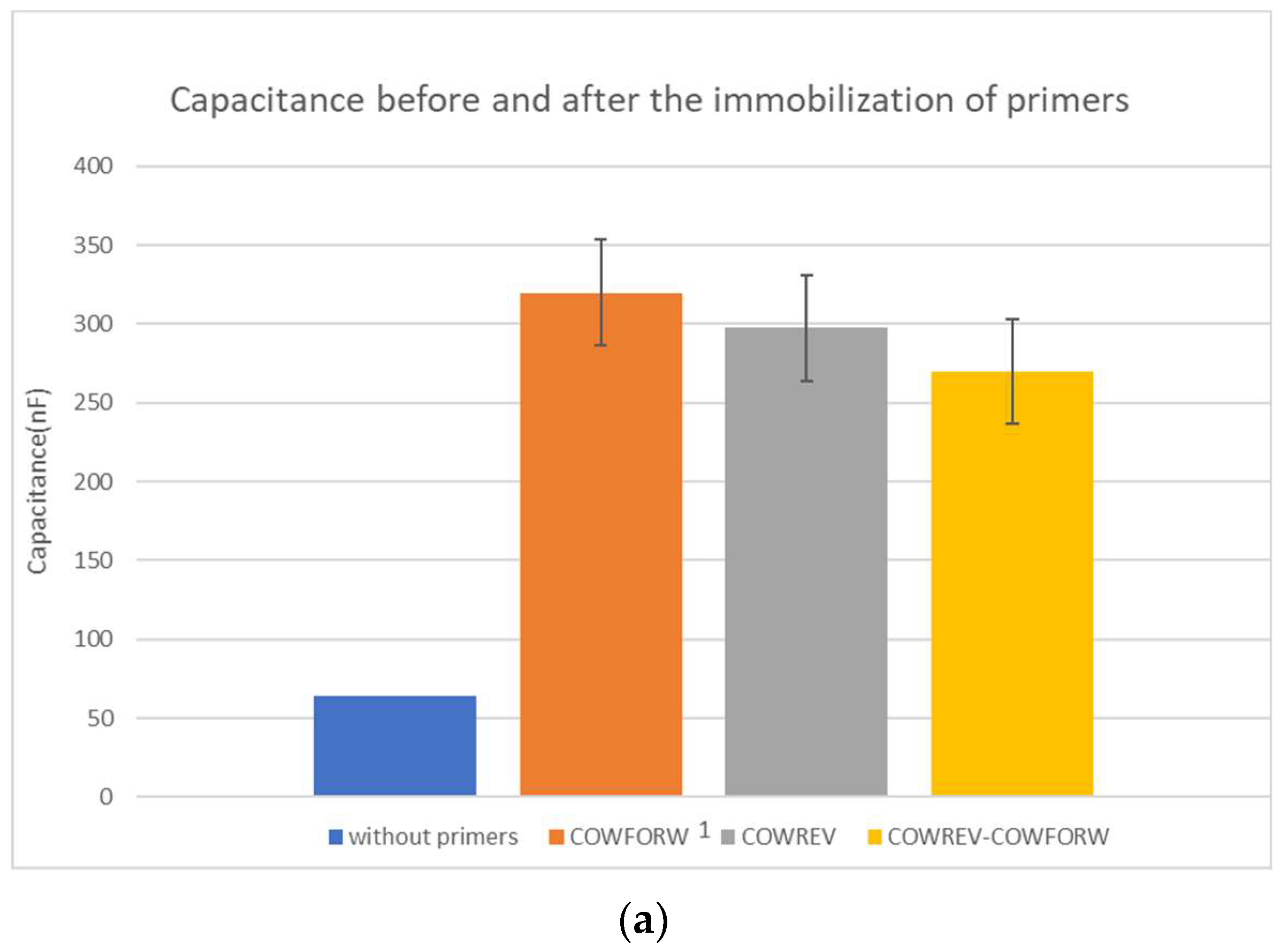
Firstly, in Table 1, the change in capacitance due to the primers’ immobilization is presented. Each column shows if there was only one primer immobilized or both of them. Due to the fact that the final values are slightly different, apart from the mean values indicating the measured capacitance after the immobilization process with 30 μL of PBS at the surface of the capacitor and the standard deviation, the mean value of the capacitance after the immobilization to the capacitance before the immobilization was also calculated. The capacitance before the immobilization with 30 μL of PBS placed at the surface of the capacitor was 63.9 nF.

Table 1.
Capacitance change after the immobilization of the primers.
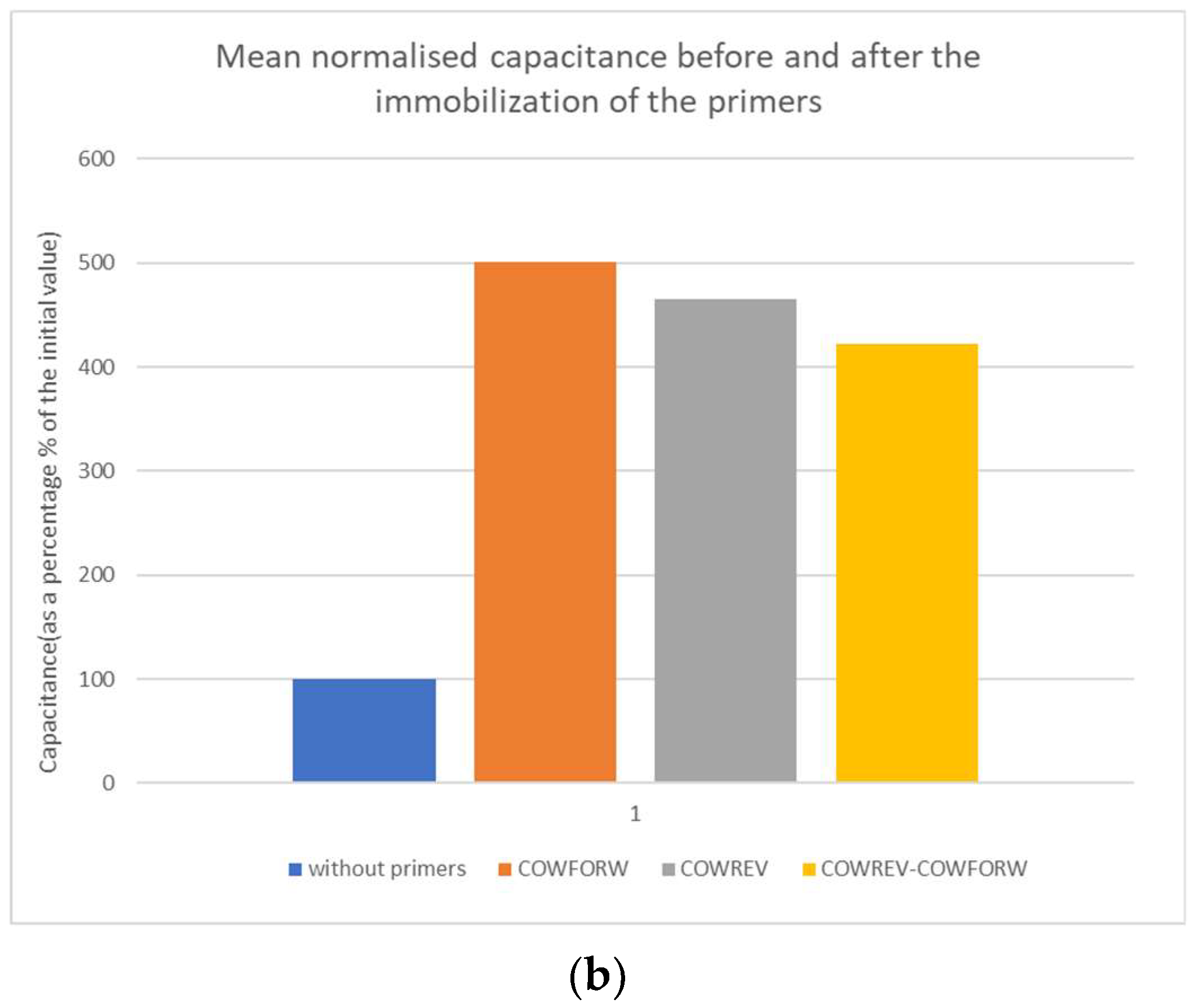
In Figure 2, there is the schematic representation of the change in capacitance in absolute and in normalized value.
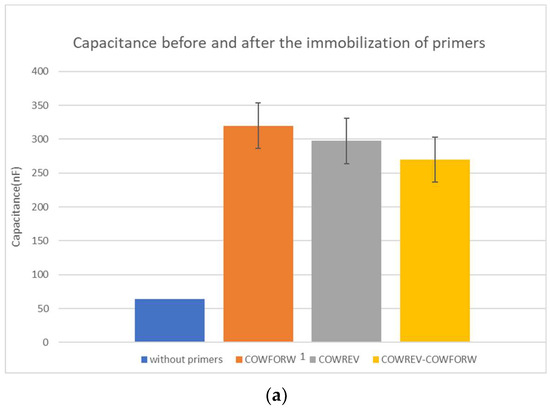
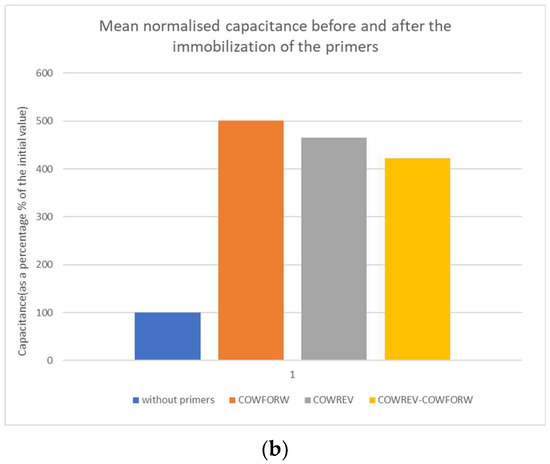
Figure 2.
(a) Mean capacitance and (b) mean normalized capacitance before and after the immobilization of the primers.
The changes in capacitance due to the immobilization of primers are significant since they roughly increase between 300 and 400%. The normalized capacitance values were used to compare the biosensor performance due to the small variation in capacitance between the primers and the combination of them.
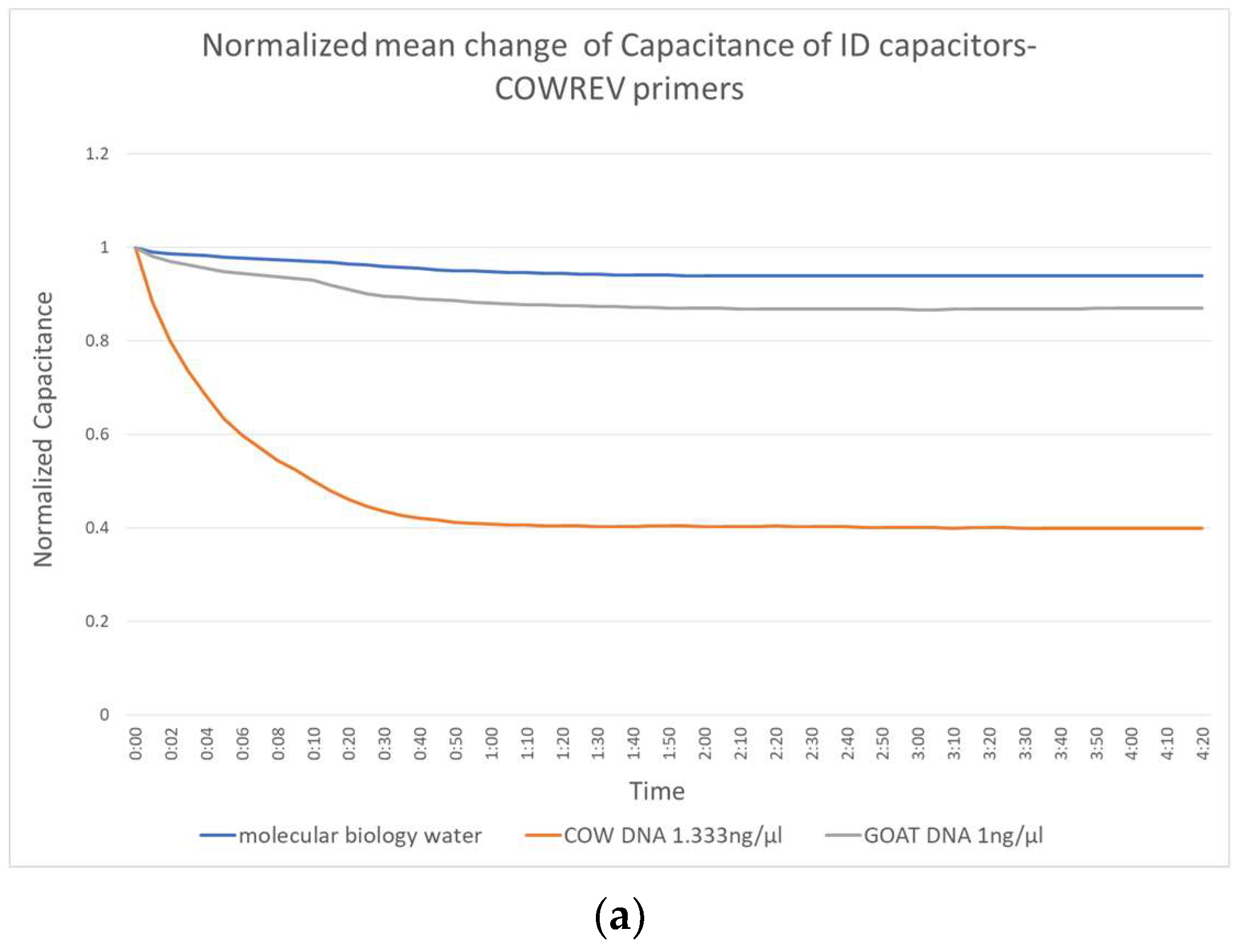
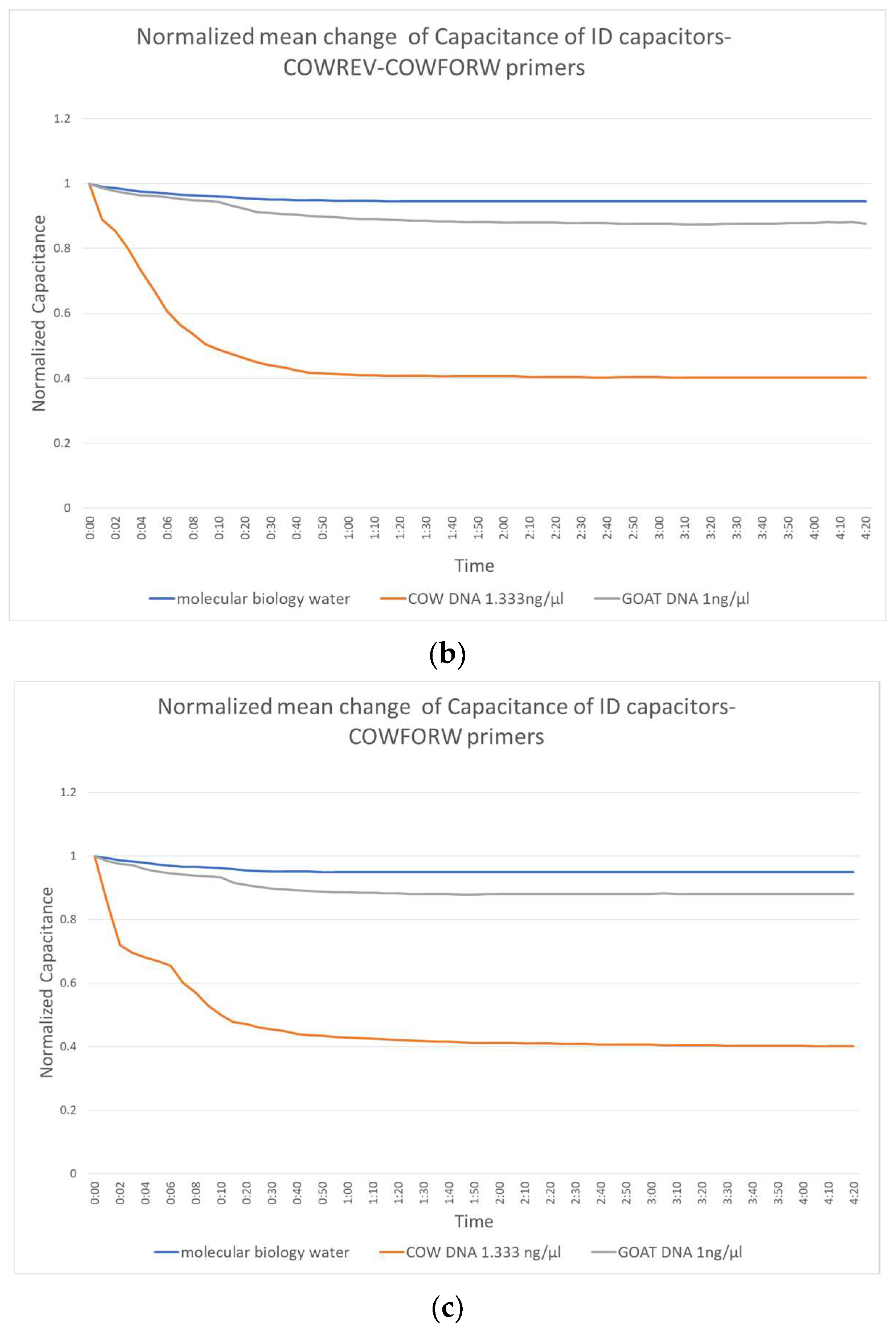
Figure 3 shows the change in capacitance caused by the application of molecular biology water, the non-complementary goat DNA and the target complementary cow DNA. Figure 3a shows the performance of the biosensor constructed with the reverse primers, Figure 3b shows those with the combination forward and reverse and Figure 3c shows the performance of the forward primer.
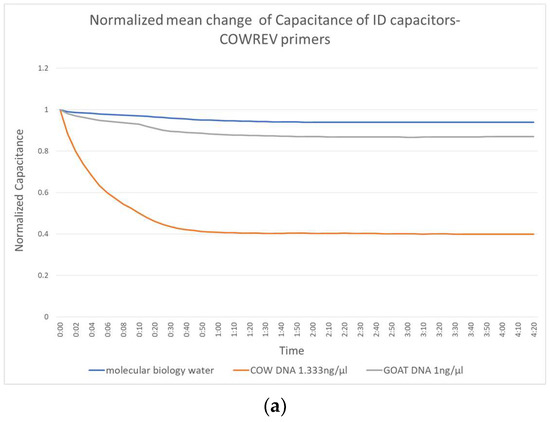
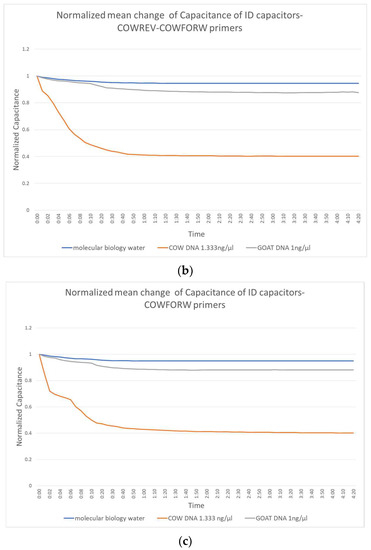
Figure 3.
Normalized mean change of IDE capacitors with (a) COWREV, (b) both COWREV and COWFORW and (c) COWFORW primers immobilized on their surface.
There is a significant distinction of the curves depending on whether the target DNA is included or not. This distinction can be observed as early as the first 10 s of the experiment, which makes the biosensor really fast. The behavior of the capacitor seems to be the same regardless of the primer used and reaches a stable value in less than 1 min.
Further development of this biosensor could include the replacement of DNA primers with PNAs (peptide nucleic acids), which have remarkable stability and, therefore, could contribute to a highly sensitive and reliable device for the detection of adulteration in milk and the protection of public health.
As was previously stated, the distinction between milk from different sources is a difficult process. Moreover, manufacturers have gained a comprehensive knowledge of the fundamental principles underlying the identification of adulteration, which has enabled them to adjust their blends to conform to the specifications of natural products in numerous instances. Consequently, the task at hand is to devise uncomplicated and economical methods for identifying adulteration that can be applied with a high level of consistency [4]. This need pushes for alternatives to the traditional methods, which are complicated and slower.
The most common practices to evaluate milk adulteration are protein-based techniques which, in recent years, have been integrated with mass spectrometry. However, they require costly equipment and specialized personnel [13] and sometimes present limitations in the distinction of bovine and goat milk [14].
Other methodologies that rely on DNA markers are different typologies of PCR that, despite their high sensitivity, have moderate cost of equipment and require complicated procedures [13].
It is also important to mention that current user-friendly biosensors, despite the low cost and the potential of portability, present higher detection limits [13].
The proposed biosensor has the potential to overcome the weakness of state-of-the-art biosensors concerning the low concentration’s limit, thanks to the fact that it utilizes a sensitive biological property of the DNA. In addition, the cost is kept low and the procedure is easy to perform and significantly fast.
4. Conclusions
Milk adulteration is a very serious, dangerous and unfortunately frequent phenomenon and, therefore, it is important to develop precise, sensitive and cheap methods in order to validate dairy products in situ. The proposed biosensor combines DNA hybridization, which is a very specific biological reaction, with the properties of interdigitated gold electrode capacitors. The results show that this biosensor has the potential to be a low-cost, easy-to-perform and fast method for the detection of milk adulteration. Further experiments in lower concentrations of the target DNA can be forecast in order for the procedure to become even simpler. In this way, the DNA extraction step can be avoided and the milk sample can be applied directly on the biosensor.
Author Contributions
D.K. performed experiments and wrote the first draft. A.G. co-conceived the biosensor, supervised the experiments and reviewed and edited the original draft. D.P.H. co-conceptualized the study and conducted experiments. A.F. reviewed and edited the original draft, co-conceptualized the study and methodology and supervised the experiments. E.H. supervised and coordinated the whole work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagraik, R.; Sharma, A.; Kumar, D.; Chawla, P.; Kumar, A.P. Milk adulterant detection: Conventional and biosensor based approaches: A review. Sens. Bio-Sens. Res. 2021, 33, 100433. [Google Scholar] [CrossRef]
- Sakti, S.P.; Chabibah, N.; Ayu, S.P.; Padaga, M.C.; Aulanni’am, A. Development of QCM Biosensor with Specific Cow Milk Protein Antibody for Candidate Milk Adulteration Detection. J. Sens. 2016, 2016, 1807647. [Google Scholar] [CrossRef]
- Renny, E.; Daniel, D.; Krastanov, A.; Zachariah, C.; Elizabeth, R. Enzyme Based Sensor for Detection of Urea in Milk. Biotechnol. Biotechnol. Equip. 2005, 19, 198–201. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of adulteration in milk: A review. Int. J. Dairy Technol. 2016, 70, 23–42. [Google Scholar] [CrossRef]
- Hlongwane, G.N.; Dodoo-Arhin, D.; Wamwangi, D.; Daramola, M.O.; Moothi, K.; Iyuke, S.E. DNA hybridisation sensors for product authentication and tracing: State of the art and challenges. S. Afr. J. Chem. Eng. 2018, 27, 16–34. [Google Scholar] [CrossRef]
- Kowalczyk, A. Trends and perspectives in DNA biosensors as diagnostic devices. Curr. Opin. Electrochem. 2020, 23, 36–41. [Google Scholar] [CrossRef]
- Wang, L.; Veselinovic, M.; Yang, L.; Geiss, B.J.; Dandy, D.S.; Chen, T. A sensitive DNA capacitive biosensor using interdigitated electrodes. Biosens. Bioelectron. 2016, 87, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, N.S.; Ramli, M.M.; Abdullah, M.M.A.B.; Halin, D.S.C.; Isa, S.S.M.; Talip, L.F.A.; Danial, N.S.; Murad, S.A.Z. Interdigitated electrodes as impedance and capacitance biosensors: A review. AIP Conf. Proc. 2017, 1885, 020276. [Google Scholar] [CrossRef]
- Georgas, A.; Lampas, E.; Houhoula, D.; Skoufias, A.; Patsilinakos, S.; Tsafaridis, I.; Patrinos, G.; Adamopoulos, N.; Ferraro, A.; Hristoforou, E. ACE2-based capacitance sensor for rapid native SARS-CoV-2 detection in biological fluids and its correlation with real-time PCR. Biosens. Bioelectron. 2022, 202, 114021. [Google Scholar] [CrossRef] [PubMed]
- Georgas, A.; Agiannis, K.; Papakosta, V.; Priftis, P.; Angelopoulos, S.; Ferraro, A.; Hristoforou, E. A Biosensor Platform for Point-of-Care SARS-CoV-2 Screening. Biosensors 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.M.L.; Tsouknidas, C.; Pytel, S.; Papatheodorou, S.; Vougiouklaki, D.; Tsakni, A.; Antonopoulos, D.; Tsakali, E.; Van Impe, J.; Houhoula, D. Effectual Gold Nanoprobe Sensor for Screening Cow Milk Adulteration in Goat Milk—Comparison with Conventional PCR. J. Agric. Sci. 2021, 13, 41. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Mafra, I.; Honrado, M.; Amaral, J.S. Animal Species Authentication in Dairy Products. Foods 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Birse, N.; Quinn, B.; Montgomery, H.; Di Wu, D.; da Silva, G.R.; van Ruth, S.M.; Elliott, C.T. Identification of milk from different animal and plant sources by desorption electrospray ionisation high-resolution mass spectrometry (DESI-MS). NPJ Sci. Food 2022, 6, 14. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).