Hydrogel-Coated Nanonet-Based Field-Effect Transistors for SARS-CoV-2 Spike Protein Detection in High Ionic Strength Samples †
Abstract
:1. Introduction
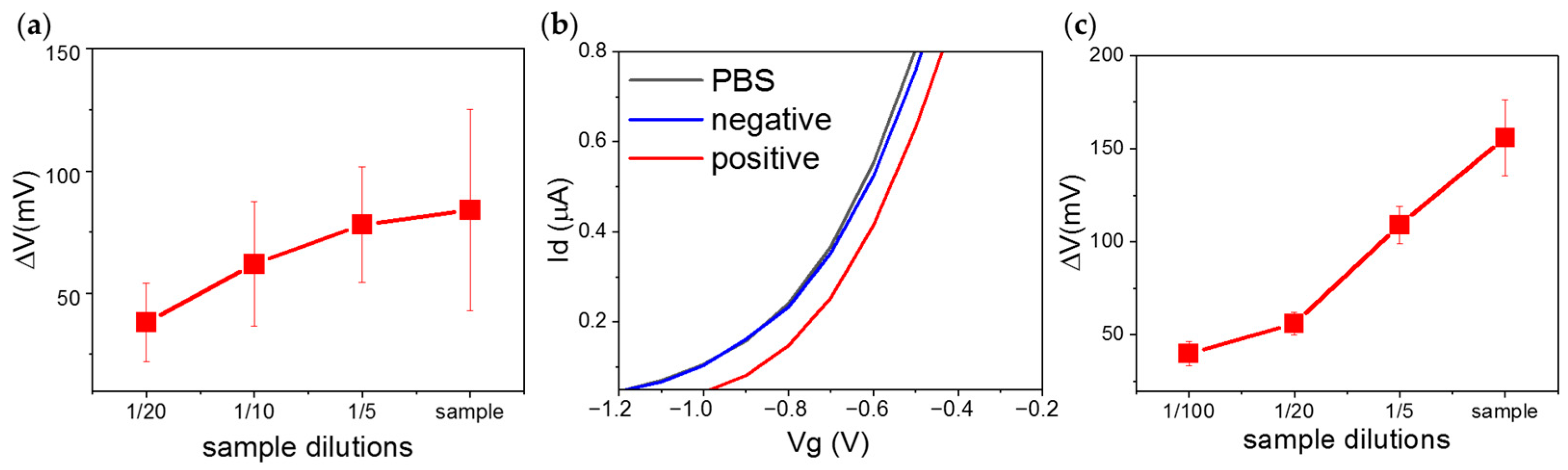
2. Results and Discussion
3. Conclusions
4. Methods
4.1. Silicon Nanonet-Based Field-Effect Transistor Fabrication
4.2. Hydrogel Preparation and Deposition
4.3. Optical Microscopy and Thickness Estimation
4.4. Virus Culture and Deactivation
4.5. Electrical Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasmi, Y.; Li, X.; Khan, J.; Ozer, T.; Choi, J.R. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: Current progress, challenges, and future prospects. Anal. Bioanal. Chem. 2021, 413, 4137–4159. [Google Scholar] [CrossRef]
- Vásquez, V.; Navas, M.-C.; Jaimes, J.A.; Orozco, J. SARS-CoV-2 electrochemical immunosensor based on the spike-ACE2 complex. Anal. Chim. Acta 2022, 1205, 339718. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Development of a Low-Cost Cotton-Tipped Electrochemical Immunosensor for the Detection of SARS-CoV-2. Anal. Chem. 2021, 93, 1826–1833. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Karnaushenko, D.; Ibarlucea, B.; Lee, S.; Lin, G.; Baraban, L.; Pregl, S.; Melzer, M.; Makarov, D.; Weber, W.M.; Mikolajick, T.; et al. Light Weight and Flexible High-Performance Diagnostic Platform. Adv. Healthc. Mater. 2015, 4, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Ibarlucea, B.; Fawzul Akbar, T.; Kim, K.; Rim, T.; Baek, C.-K.; Ascoli, A.; Tetzlaff, R.; Baraban, L.; Cuniberti, G. Ultrasensitive detection of Ebola matrix protein in a memristor mode. Nano Res. 2018, 11, 1057–1068. [Google Scholar] [CrossRef]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye Screening Length on Nanowire Field Effect Transistor Sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.-M.; Lieber, C.M. General Strategy for Biodetection in High Ionic Strength Solutions Using Transistor-Based Nanoelectronic Sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Prince, T.; Anderson, E.R.; Casas-Sanchez, A.; Smith, S.L.; Cansado-Utrilla, C.; Solomon, T.; Griffiths, M.J.; Acosta-Serrano, Á.; Turtle, L.; et al. Methods of Inactivation of SARS-CoV-2 for Downstream Biological Assays. J. Infect. Dis. 2020, 222, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Chwalek, K.; Prokoph, S.; Zieris, A.; Levental, K.R.; Freudenberg, U.; Werner, C. Defined Polymer–Peptide Conjugates to Form Cell-Instructive starPEG–Heparin Matrices In Situ. Adv. Mater. 2013, 25, 2606–2610. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parichenko, A.; Choi, W.; Shin, S.; Stadtmüller, M.; Akbar, T.F.; Werner, C.; Lee, J.-S.; Ibarlucea, B.; Cuniberti, G. Hydrogel-Coated Nanonet-Based Field-Effect Transistors for SARS-CoV-2 Spike Protein Detection in High Ionic Strength Samples. Eng. Proc. 2023, 35, 11. https://doi.org/10.3390/IECB2023-14566
Parichenko A, Choi W, Shin S, Stadtmüller M, Akbar TF, Werner C, Lee J-S, Ibarlucea B, Cuniberti G. Hydrogel-Coated Nanonet-Based Field-Effect Transistors for SARS-CoV-2 Spike Protein Detection in High Ionic Strength Samples. Engineering Proceedings. 2023; 35(1):11. https://doi.org/10.3390/IECB2023-14566
Chicago/Turabian StyleParichenko, Alexandra, Wonyeong Choi, Seonghwan Shin, Marlena Stadtmüller, Teuku Fawzul Akbar, Carsten Werner, Jeong-Soo Lee, Bergoi Ibarlucea, and Gianaurelio Cuniberti. 2023. "Hydrogel-Coated Nanonet-Based Field-Effect Transistors for SARS-CoV-2 Spike Protein Detection in High Ionic Strength Samples" Engineering Proceedings 35, no. 1: 11. https://doi.org/10.3390/IECB2023-14566
APA StyleParichenko, A., Choi, W., Shin, S., Stadtmüller, M., Akbar, T. F., Werner, C., Lee, J.-S., Ibarlucea, B., & Cuniberti, G. (2023). Hydrogel-Coated Nanonet-Based Field-Effect Transistors for SARS-CoV-2 Spike Protein Detection in High Ionic Strength Samples. Engineering Proceedings, 35(1), 11. https://doi.org/10.3390/IECB2023-14566










