1. Introduction
Continuous irradiation causes photodamage and phototoxicity when imaging living samples and specimens. In order to overcome this problem, light sheet fluorescence microscopy (LSFM) was developed as an imaging technique with good optical sectioning, to record faster and scan larger sample volumes at low radiation intensities over longer time frames [
1]. The LSFM is similar to laser scanning confocal microscopy (LSCM) [
2], but the emitted fluorescence light is collected by the detection objective in a perpendicular direction from the excitation light and without the need for a confocal aperture. In LSFM, the excitation light does not pass through the entire sample; instead, the sample is illuminated from the side with a thin light sheet (LS) beam. This clever idea allows the LSFM to collect the emitted light from fluorophores localized in the thin LS plane only and not from fluorophores belonging outside the LS plane. This results in a low light dose usage, and therefore reduces both photobleaching and phototoxicity [
3,
4,
5]. LSFM is also referred to as selective-plane illumination microscopy (SPIM) when imaging large samples [
1]. The axial resolution is given by both the thickness of the LS (ideally it is extremely thin) and the detection NA [
6,
7]. If we choose a microscope objective with an NA of 1.1, we can obtain a very thin light sheet. However, the light sheet confocal length, over which the sheet is thin, must be matched to the FOV of the recorded images or the size of the samples. The thinner the sheet (and higher the NA), the shorter the thin region is.
In most of the cases, the light sheet has a Gaussian profile at the specimen due to the Gaussian shape of the laser beams. A thicker Gaussian light sheet can cover a larger FOV but has a lower resolution and optical sectioning. Non-Gaussian beams using arrays of Bessel beams, called “lattice” beams, have been employed to generate images with excellent scanning efficiency and resolution, by using very thin light sheets. The lattice light sheet microscopy (LLSM) system uses the LSFM principle with an optical lattice created with Bessel beams, resulting in a light sheet with a thickness of ~400 nm [
8]. The typical acquisition rate of an LLSM system is hundreds of frames per second, making LLSM the ultimate tool for live-cell fluorescence imaging.
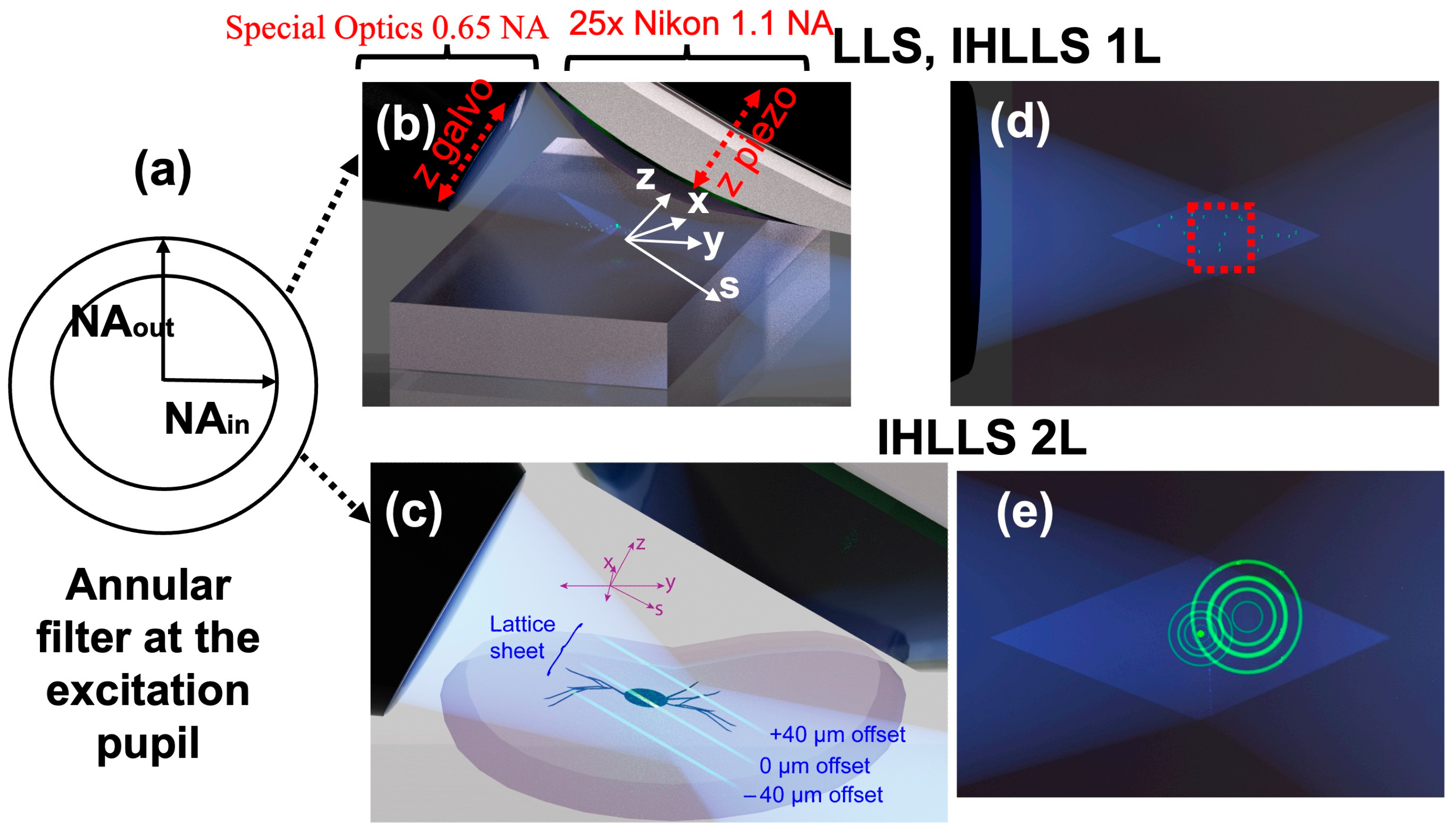
The lattice light sheet,
Figure 1a, is formed by superimposing a linearly polarized sheet of light with a binary phase map of Bessel beams on a binary spatial light modulator (SLM), which is conjugated to the image plane of the excitation objective. Before reaching the sample plane, the beam passes a Fraunhofer lens and is further projected onto a transparent optical annulus,
Figure 1b, to eliminate unwanted diffraction orders and lengthen the light sheet. In dithered mode, a 2D lattice pattern is oscillated in the x-axis using a galvanometer (x-galvo), to create a uniform sheet, while another galvanometer mirror moves together with the detection objective, in the z-axis (z-galvo), to scan the sample volume.
A more recent approach of the LLSM is the new detection path,
Figure 1c,d, that uses the incoherent holography idea, called an incoherent holographic lattice light sheet (IHLLS) [
9], to scan the sample volume without moving the detection objective. It is possible by projecting two diffractive lenses at various z-galvo positions, using a phase SLM, to encode the depth position of the sample being imaged. The annular mask,
Figure 1b, mentioned above, was centered on an anulus with a higher NA (HNA), NAout = 0.5, NAin = 0.485, and a sheet length 15 μm; therefore, the beams used for excitation were more Bessel-like beams. The intensity profile of the obtained optical lattice is determined by both the applied binary phase map and the geometry of the transparent annulus.
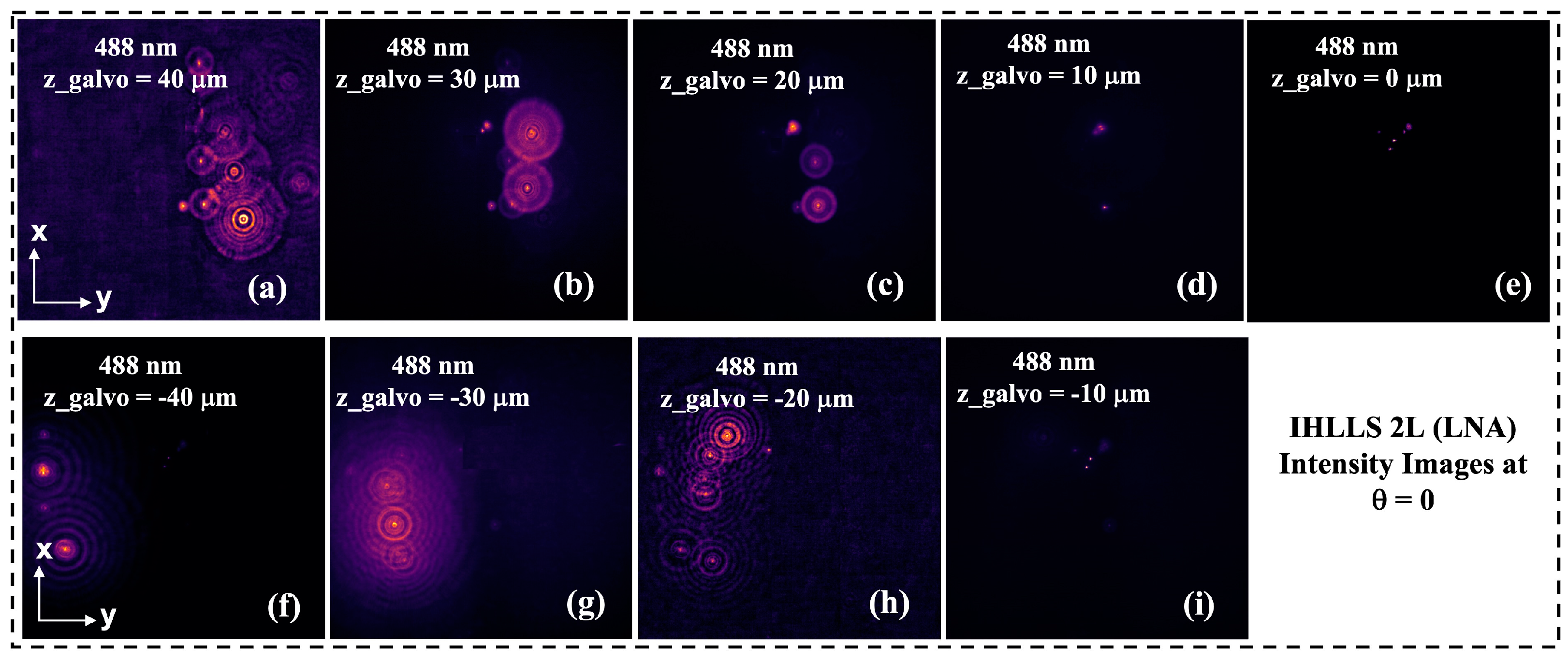
Here, we propose to expand our previous IHLLS detection technique that uses a HNA by choosing a diffractive mask annulus with a lower NA (LNA). In this case, the anulus has the following parameters: NAout = 0.3, NAin = 0.275, and sheet length 75 μm. The new detection approach uses a Gaussian-like excitation beam similar to conventional light sheet systems. This provides a tradeoff between the size of the imaged beam and the resolution of the ultimate image in the z-plane. This has advantages for imaging objects at larger intercellular scales.
2. Imaging Performances and Diffraction Mask Parameter Selection
In LLS, near-non-diffracting Bessel beams are created at the focal position of the excitation objective, by projecting an annular excitation beam,
Figure 2a, in the back focal plane of the excitation objective. While the z-galvo and z-piezo are moved along the z axis to acquire stacks in LLS/IHLLS 1L,
Figure 2b, in IHLLS 2L only the z-galvo is moved at various z positions,
Figure 2c. The imaging area/volume is limited to a small FOV,
Figure 2d red square, due to the tradeoff between the sheet’s propagation length and its thickness. Although IHLLS 1L is the incoherent version of the LLS detection module, the axial resolution in LLS is better than the axial resolution in IHLLS 1L due to the blurry effect of the diffractive lens that focusses to infinity. The FOV limitation is no longer an issue in IHLLS 2L because the combination of the z-galvo motion in the range
and the dual diffractive lenses uploaded onto the phase SLM allows scanning for the whole FOV of the CMOS detector, which is 208 × 208
. Moreover, the axial resolution is better in IHLLS 2L due to the fact that the focus position is found numerically.
The NA of the beam is an overall combination of the inner and outer numerical apertures (NAs) of the annular mask filter, NA
out, and NA
in. The Bessel-like beams are considered to be created using those annuli on the diffraction mask with NA
out ≥ 0.5 and the Gaussian-like beams created with the annuli, with NA
out < 0.4. The thickness of the pattern generated by these beams at the focal position of the excitation objective is given by
, and the sheet length or the FOV by
, where
,
, and n = 1.33 [
7]. Although increasing the NA difference produces a thinner light sheet illumination profile, which provides higher axial resolution, the length that the light sheet spans is reduced, and thus it is hard to further compress the light sheet thickness while maintaining a relatively large FOV. The sheet parameters of the two diffraction mask filters at 488 nm are w
sheet-HNA = 443.6 nm, w
sheet-LNA = 813.3 nm, and the FWHM sheet lengths are FWHM
sheet-HNA = 15 μm and FWHM
sheet-LNA = 75 μm.
Imaging can be done with various sheet lengths: cultured cells and intracellular events could be imaged using light sheets with lengths smaller than ~20 μm, small organoids with sheets of lengths ~30 to 40 μm, and large organoids with sheets of lengths ~50 to 70 μm.
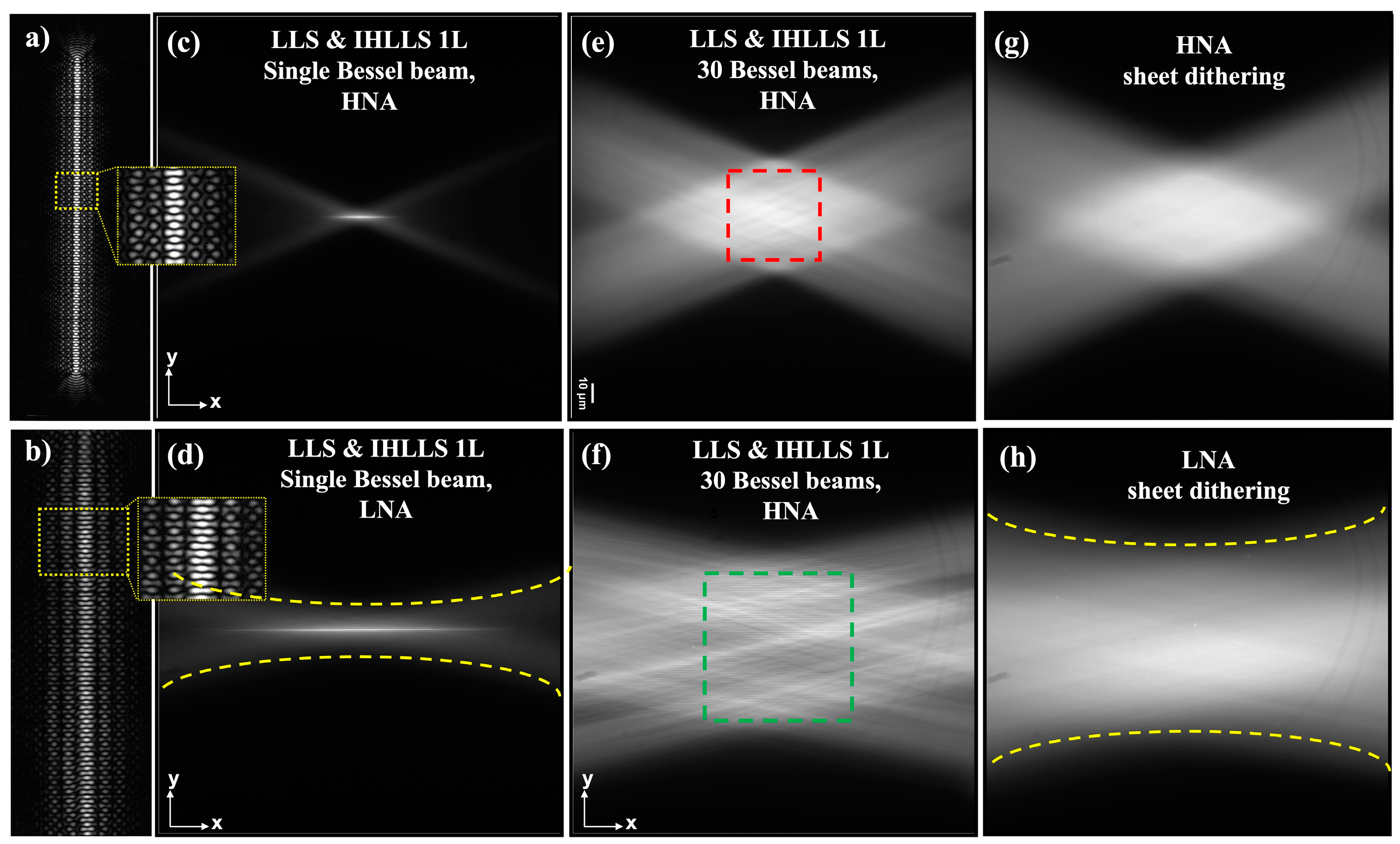
The beams generated by using the two annuli are shown in
Figure 3. The upper row shows the optical lattice generated with the HNA annulus and the bottom row the optical lattice generated by the LNA annulus. Optical lattices can be designed to balance the confinement and the thickness by adjusting the spacing between each pair of beams. As an example, the lattice in
Figure 3a is designed as a coherent superposition of 30 Bessel beams with a spacing of 0.99. Similarly, the lattice in
Figure 3b is designed by using 26 Bessel beams with a spacing of 1.755. A single Bessel beam is shown in
Figure 3c,d, respectively. We can see that the lower NA beam has a Gaussian-like shape. The beams at the sample plane, corresponding to the patterns in
Figure 3a,b, are shown in
Figure 3e,f, and the same beams but with the dithered mode are shown in
Figure 3g,h.