Zinc-Ion Battery on a Polyester-Cotton Textile †
Abstract
:1. Introduction
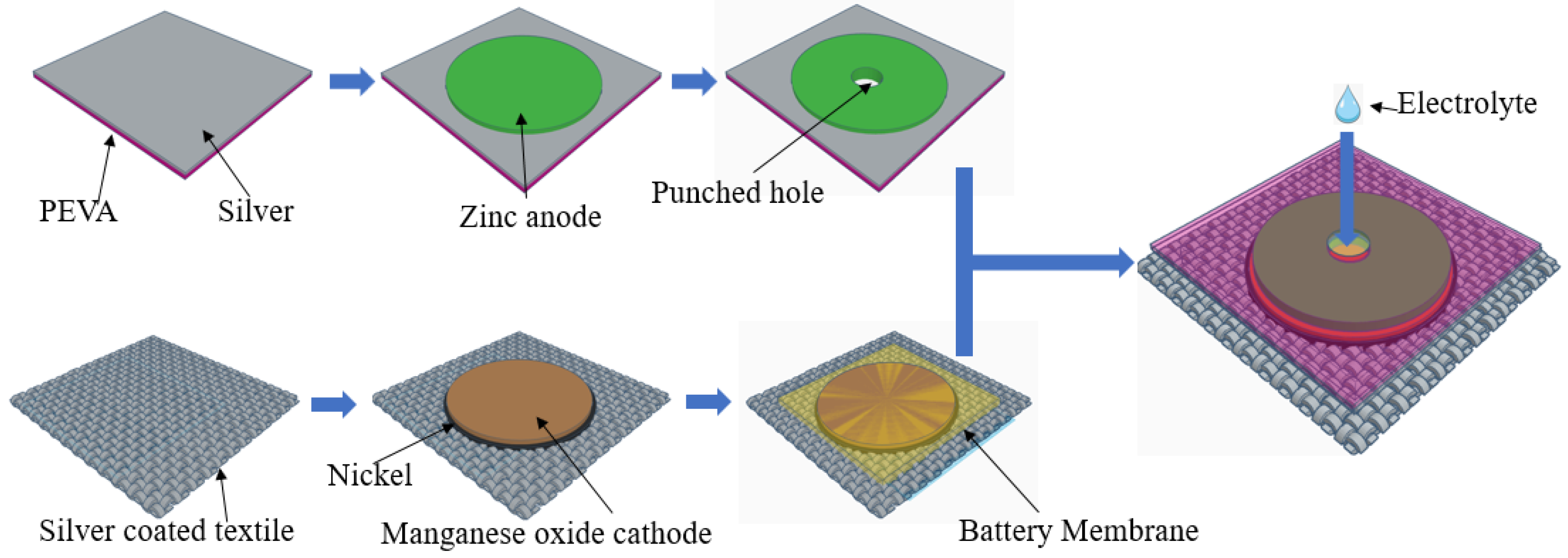
2. Material and Methods
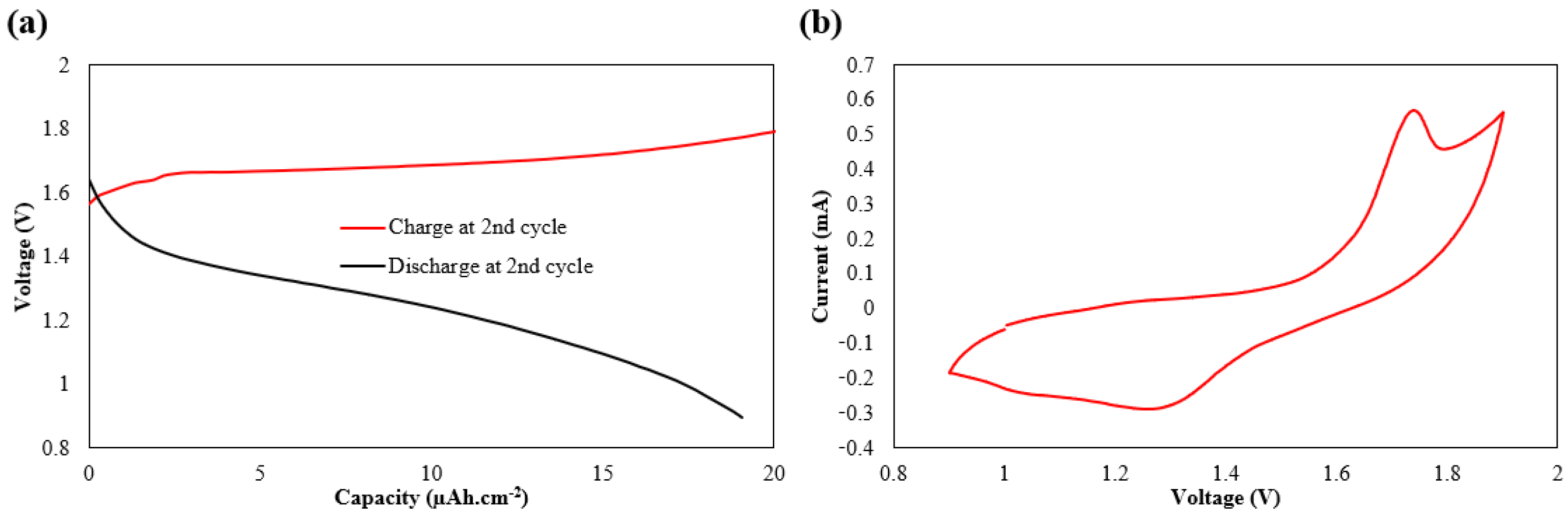
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, D.; Beeby, S.; Tudor, J.; Harris, N. A credit card-sized self-powered smart sensor node. Sens. Actuators A Phys. 2011, 169, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.G.; da Silva, J.M. A transceiver for E-textile body-area-networks. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisbon, Portugal, 11–12 June 2014; pp. 1–6. [Google Scholar] [CrossRef] [Green Version]
- Beeby, S.; Zhu, D. Energy Harvesting Products and Forecast. Mater. Sci. Found. 2011, 9, 197–218. [Google Scholar]
- Dias, T. Electronic Textiles Smart Fabrics and Wearable Technology; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Yong, S.; Hillier, N.; Beeby, S. Fabrication of a Flexible Aqueous Textile Zinc-Ion Battery in a Single Fabric Layer. Front. Electron. 2022, 3, 866527. [Google Scholar] [CrossRef]
- Xu, N.; Yan, C.; He, W.; Xu, L.; Jiang, A.; Zheng, A.; Wu, H.; Chen, M.; Diao, G. Flexible electrode material of V2O5 carbon fibre cloth for enhanced zinc ion storage performance in the flexible zinc-ion battery. J. Power Sources 2022, 533, 231358. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, S.; Hillier, N.; Beeby, S. Zinc-Ion Battery on a Polyester-Cotton Textile. Eng. Proc. 2023, 30, 20. https://doi.org/10.3390/engproc2023030020
Yong S, Hillier N, Beeby S. Zinc-Ion Battery on a Polyester-Cotton Textile. Engineering Proceedings. 2023; 30(1):20. https://doi.org/10.3390/engproc2023030020
Chicago/Turabian StyleYong, Sheng, Nick Hillier, and Steven Beeby. 2023. "Zinc-Ion Battery on a Polyester-Cotton Textile" Engineering Proceedings 30, no. 1: 20. https://doi.org/10.3390/engproc2023030020
APA StyleYong, S., Hillier, N., & Beeby, S. (2023). Zinc-Ion Battery on a Polyester-Cotton Textile. Engineering Proceedings, 30(1), 20. https://doi.org/10.3390/engproc2023030020






