Success and Failure in Antibody Recognition by Surface-Type Sensors: Essential Prerequisites †
Abstract
1. Introduction
2. Methods
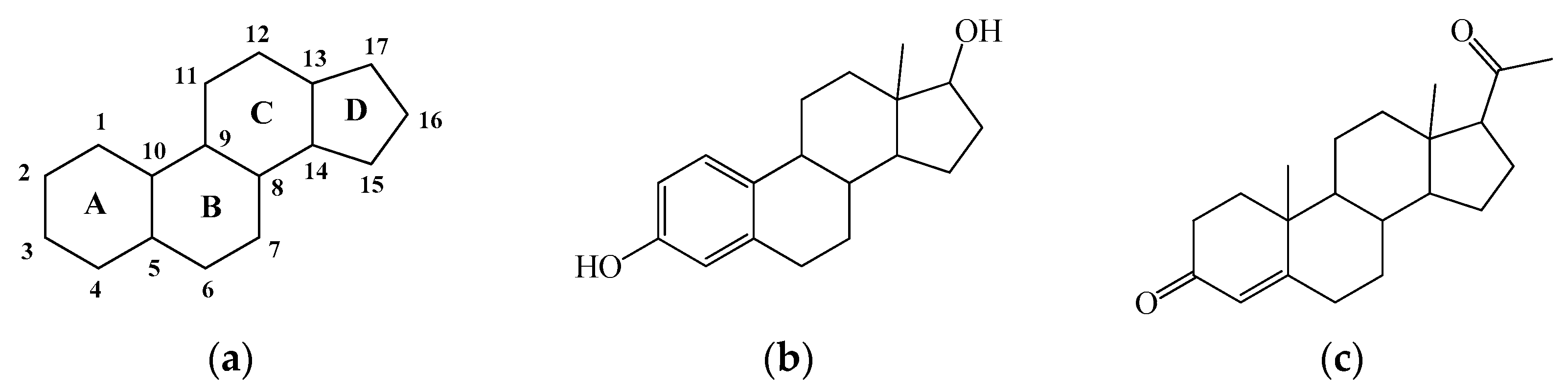
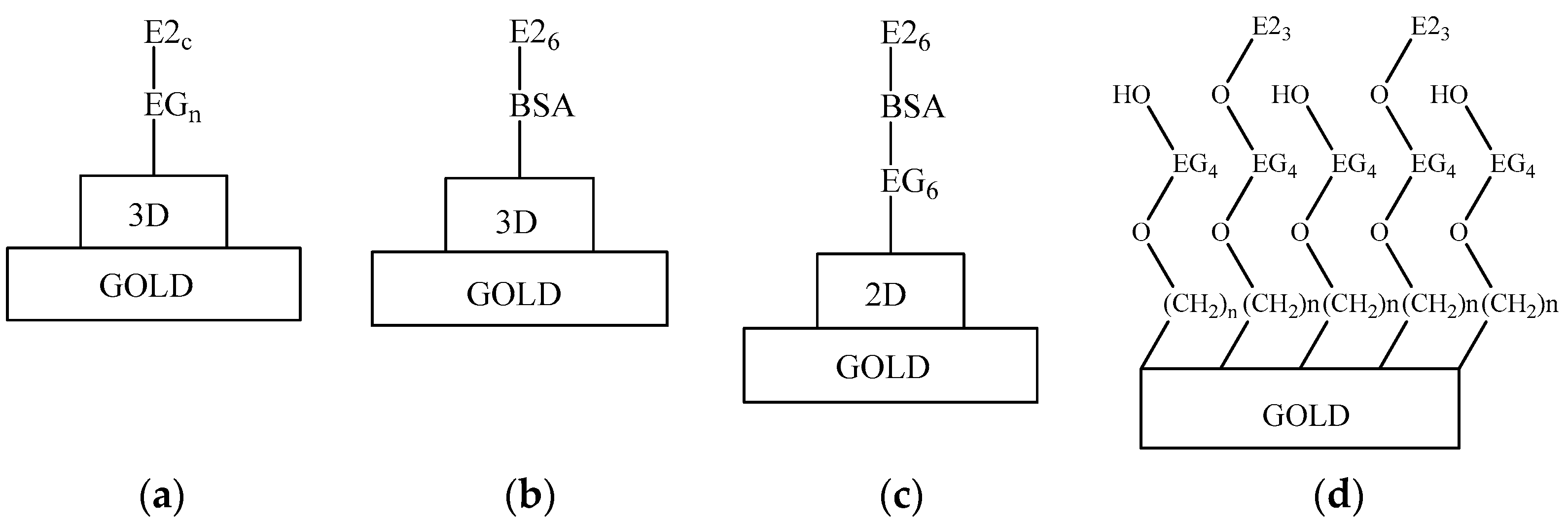
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, J.R.; Sanjoy, C.; Chakraborty, T.R. Estrogen-like endocrine disrupting chemicals affecting puberty in humans. Med. Sci. Monit. 2009, 15, RA137–RA145. [Google Scholar] [PubMed]
- Tian, W.; Wang, L.; Lei, H.; Sun, Y.; Xiao, Z. Antibody production and application for immunoassay development of environmental hormones. Chem. Biol. Technol. Agric. 2018, 5, 5. [Google Scholar] [CrossRef]
- O’Kennedy, R.; Murphy, C. (Eds.) Immunoassays. In Development, Applications and Future Trends, 1st ed.; Imprint Jenny Stanford Publishing: New York, NY, USA, 2017; p. 470. [Google Scholar]
- Homola, J. Surface Plasmon Resonance Based Sensors; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2006; p. 251. [Google Scholar]
- Mitchell, J. Small Molecule Immunosensing Using Surface Plasmon Resonance. Sensors 2010, 10, 7323–7346. [Google Scholar] [CrossRef] [PubMed]
- Hanselman, T.A.; Graetz, D.A.; Wilkie, A.C. Comparison of three enzyme immunoassays for measuring 17beta-estradiol in flushed dairy manure wastewater. J. Environ. Qual. 2004, 33, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Farre, M.; Kuster, M.; Brix, R.; Rubio, F.; Lopez de Alda, M.J.; Barcelo, D. Comparative study of an estradiol enzyme-linked immunosorbent assay kit, liquid chromatography-tandem mass spectrometry, and ultra performance liquid chromatography-quadrupole time of flight mass spectrometry for part-per-trillion analysis of estrogens in water samples. J. Chromatogr. A 2007, 1160, 166–175. [Google Scholar]
- Yucel, N.; Madenci, O.C.; Boluk, A.; Koroglu, L.; Temel, Y.; Orcun, A. A Comparison of Three Widely Used Immunoassay Systems in Cortisol Measurement. Clin. Lab. 2015, 61, 1947–1952. [Google Scholar] [CrossRef]
- Konkle, A.T.; McCarthy, M.M. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 2011, 152, 223–235. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Miyakawa, I.; Soares, J.R.; Goebelsmann, U. Radioimmunoassay of estriol-16-glucuronide using tritiated and radioiodinated radioligands: Direct radioimmunoassay of urinary estriol-16-glucuronide during the menstrual cycle. J. Steroid Biochem. 1979, 10, 443–448. [Google Scholar] [CrossRef]
- Tschop, M.; Behre, H.M.; Nieschlag, E.; Dressendorfer, R.A.; Strasburger, C.J. A time-resolved fluorescence immunoassay for the measurement of testosterone in saliva: Monitoring of testosterone replacement therapy with testosterone buciclate. Clin. Chem. Lab. Med. 1998, 36, 223–230. [Google Scholar] [CrossRef]
- Thomas, C.M.; van den Berg, R.J.; Segers, M.F.; Geelen, L.M.; Hollanders, J.M.; Doesburg, W.H.; Houx, P.C. Time-resolved fluoroimmunoassay for unconjugated estrogen in urine: Comparison with a fluorometric assay for total estrogen and application in an in vitro fertilization program. Clin. Chem. 1990, 36, 1774–1778. [Google Scholar] [CrossRef]
- Tan, C.; Schenk, J.A.; Gajovic-Eichelmann, N.; Sellrie, F.; Bier, F.F. A new one-step antigen heterologous homogeneous fluorescence immunoassay for progesterone detection in serum. Talanta 2015, 134, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.B.; Chen, H.; Lin, Z.; Liang, S.X.; Lin, J.M. A secondary antibody format chemiluminescence immunoassay for the determination of estradiol in human serum. Talanta 2010, 82, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Westermann, J.; Demir, A.; Herbst, V. Determination of cortisol in saliva and serum by a luminescence-enhanced enzyme immunoassay. Clin. Lab. 2004, 50, 11–24. [Google Scholar] [PubMed]
- Gonzalez, A.; Avivar, J.; Cerda, V. Estrogens determination in wastewater samples by automatic in-syringe dispersive liquid-liquid microextraction prior silylation and gas chromatography. J. Chromatogr. A 2015, 1413, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.S.; Wu, Y. Surface plasmon resonance signal enhancement for immunoassay of small molecules. Methods Mol. Biol. 2010, 627, 113–129. [Google Scholar] [PubMed]
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit. Rev. Anal. Chem. 2015, 45, 97–105. [Google Scholar] [CrossRef]
- Briciu, R.D.; Kot-Wasik, A.; Namiesnik, J. Analytical Challenges and Recent Advances in the Determination of Estrogens in Water Environments. J. Chromatogr. Sci. 2009, 47, 127–139. [Google Scholar] [CrossRef]
- Hamid, H.; Eskicioglu, C. Fate of estrogenic hormones in wastewater and sludge treatment: A review of properties and analytical detection techniques in sludge matrix. Water Res. 2012, 46, 5813–5833. [Google Scholar] [CrossRef]
- Snopok, B.; Yurchenko, M.; Szekely, L.; Klein, G.; Kasuba, E. SPR based immuno-capture approach for in vitro analysis of protein complex formation: Mapping of MRS18-2 binding site on retinoblastoma protein. Anal. Bioanal. Chem. 2006, 386, 2063–2073. [Google Scholar] [CrossRef]
- Beketov, G.V.; Shirshov, Y.M.; Shynkarenko, O.V.; Chegel, V.I. Surface plasmon resonance spectroscopy: Prospects of superstate refractive index variation for separate extraction of molecular layer parameters. Sens. Actuators B 1998, 48, 432–438. [Google Scholar] [CrossRef]
- Goodrow, M.H.; Hammock, B.D. Hapten design for compound-selective antibodies: ELISAS for environmentally deleterious small molecules. Anal. Chim. Acta 1998, 376, 83–91. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Wu, Y.; Cook, C.J.; Main, L. Estrogen conjugation and antibody binding interactions in surface plasmon resonance biosensing. Steroids 2006, 71, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Shimada, T.; Miyagawa, H.; Akamatsu, M. Surface plasmon resonance-based immunoassay for 17β-estradiol and its application to the measurement of estrogen receptor-binding activity. Anal. Bioanal. Chem. 2005, 381, 667–673. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Luo, Z.; Wang, J.; Ma, D. Analysis of 17β-Estadiol from Sewage in Coastal Marine Environment by Surface Plasmon Resonance Technique. Chem. Res. Chin. Univ. 2007, 23, 404–407. [Google Scholar] [CrossRef]
- Ou, H.; Luo, Z.; Jiang, H.; Zhou, X.; Wang, H.; Song, C. Indirect Inhibitive Immunoassay for Estradiol Using Surface Plasmon Resonance Coupled to Online In-Tube SPME. Anal. Lett. 2009, 42, 2758–2773. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Mateescu, A.; Sergelen, K.; Kibrom, A.; Jonas, U.; Wei, T.; Dostalek, J. Biosensor based on hydrogel optical waveguide spectroscopy for the detection of 17b-estradiol. Talanta 2013, 104, 149–154. [Google Scholar] [CrossRef]
- Cao, Y.; McDermott, M.T. A Surface Plasmon Resonance Based Inhibition Immunoassay for Sensitive and Selective Detection of 17β-Estradiol. Anal. Biochem. 2018, 557, 7–12. [Google Scholar] [CrossRef]
- Dhar, T.K.; Samanta, A.K.; Ali, E. Homogeneous enzyme immunoassay of estradiol using estradiol-3-0-carboxymethyl ether as hapten. Steroids 1988, 51, 519–526. [Google Scholar] [CrossRef]
- Li, C.Y.; Jiang, J.Q. Development and characterization of anti-estradiol polyclonal antibody. Adv. Mater. Res. 2012, 461, 67–70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravchenko, S.; Boltovets, P.; Manoilov, E.; Poix-Shinkaruk, S.; Vellutini, L.; Snopok, B. Success and Failure in Antibody Recognition by Surface-Type Sensors: Essential Prerequisites. Eng. Proc. 2022, 27, 9. https://doi.org/10.3390/ecsa-9-13221
Kravchenko S, Boltovets P, Manoilov E, Poix-Shinkaruk S, Vellutini L, Snopok B. Success and Failure in Antibody Recognition by Surface-Type Sensors: Essential Prerequisites. Engineering Proceedings. 2022; 27(1):9. https://doi.org/10.3390/ecsa-9-13221
Chicago/Turabian StyleKravchenko, Sergii, Praskoviya Boltovets, Eduard Manoilov, Svitlana Poix-Shinkaruk, Luc Vellutini, and Borys Snopok. 2022. "Success and Failure in Antibody Recognition by Surface-Type Sensors: Essential Prerequisites" Engineering Proceedings 27, no. 1: 9. https://doi.org/10.3390/ecsa-9-13221
APA StyleKravchenko, S., Boltovets, P., Manoilov, E., Poix-Shinkaruk, S., Vellutini, L., & Snopok, B. (2022). Success and Failure in Antibody Recognition by Surface-Type Sensors: Essential Prerequisites. Engineering Proceedings, 27(1), 9. https://doi.org/10.3390/ecsa-9-13221





