Anion Dual Mode Fluoro-Chromogenic Chemosensor Based on a BODIPY Core †
Abstract
1. Introduction
2. Materials and Method
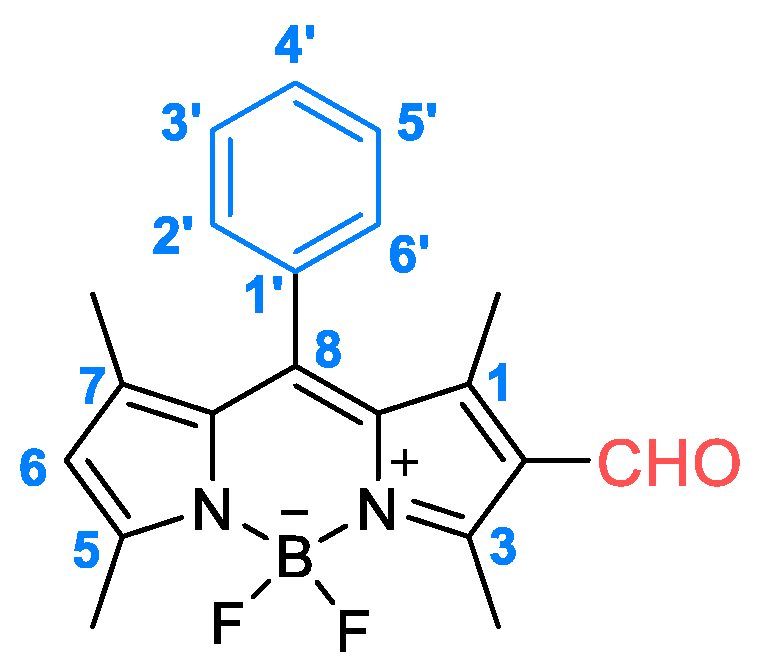
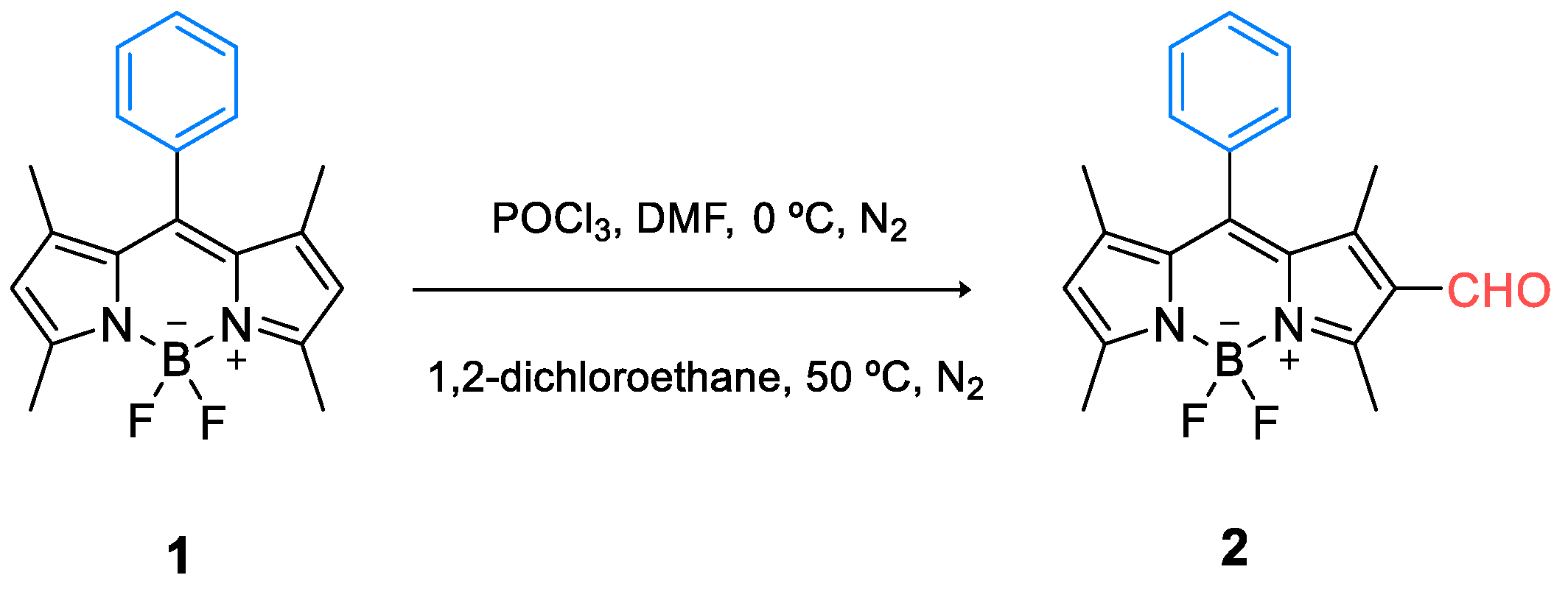
2.1. Synthesis of the BODIPY Derivative 2
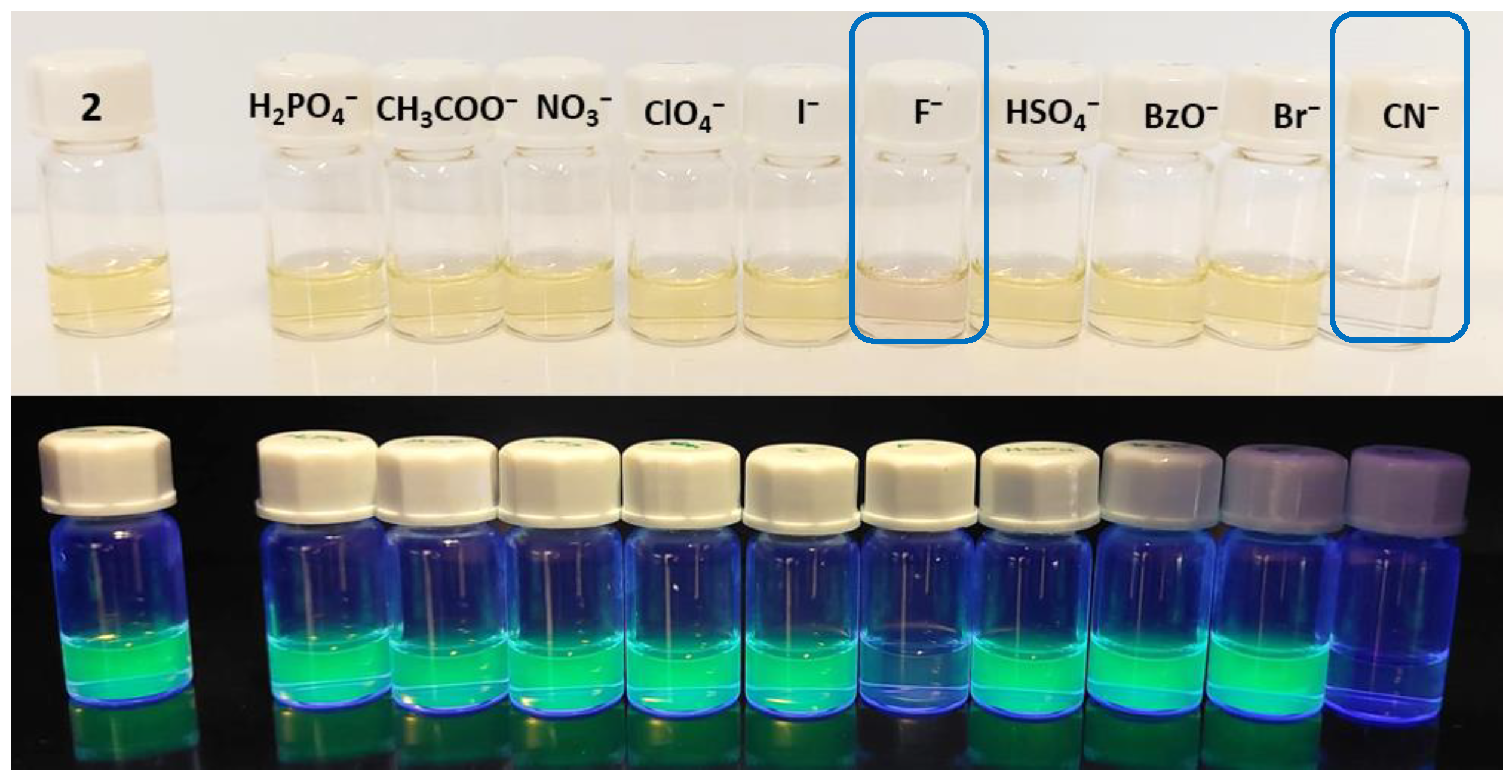
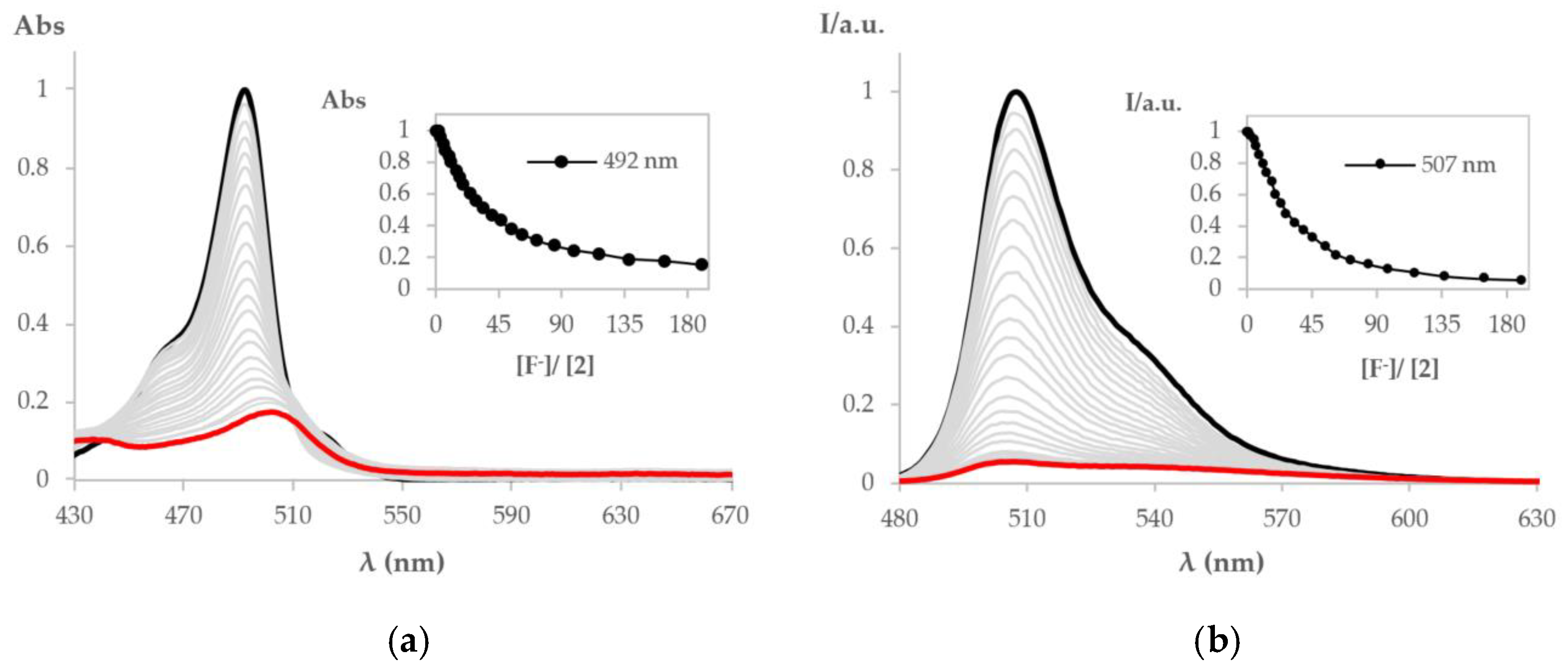
2.2. Preliminary Chemosensing Studies of BODIPY Derivative 2
3. Results and Discussion
3.1. Synthesis and Photophysical Characterization of BODIPY Derivative 2
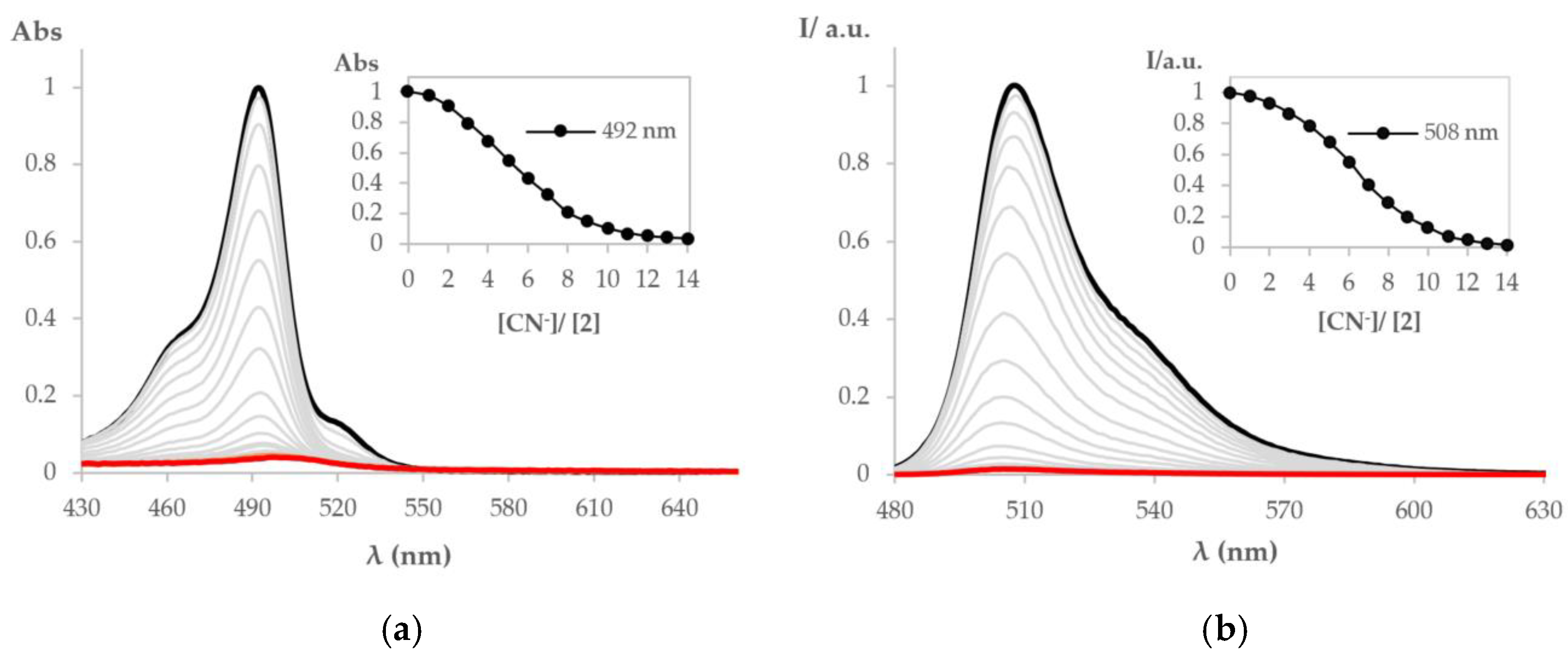
3.2. Chemosensing Studies of BODIPY Derivative 2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, G.; Fegade, U.A.; Singh, A.; Sahoo, S.K.; Kuwar, A.S.; Singh, N. Anion sensing with chemosensors having multiple NH recognition units. TrAC-Trends Anal. Chem. 2017, 95, 86–109. [Google Scholar] [CrossRef]
- Santos-Figueroa, L.E.; Moragues, M.E.; Climent, E.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Passador, K.; Goze, C.; Denat, F.; Bodio, E.; Salmain, M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord. Chem. Rev. 2018, 358, 108–124. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY dye, the most versatile fluorophore ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Collado, D.; Casado, J.; González, S.R.; Navarrete, J.T.L.; Suau, R.; Perez-Inestrosa, E.; Pappenfus, T.M.; Raposo, M.M.M. Enhanced functionality for donor–acceptor oligothiophenes by means of inclusion of BODIPY: Synthesis, electrochemistry, photophysics, and model chemistry. Chem. Eur. J. 2011, 17, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A dual channel sulphur-containing macrocycle functionalised BODIPY probe for the detection of Hg(II) in mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M. M Anion colorimetric chemosensor based on a benzimidazole-functionalized BODIPY derivative. Chem. Proc. 2022, 8, 90. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. A meso-triphenylamine-BODIPY derivative for the optical chemosensing of metal ions. Chem. Proc. 2021, 3, 65. [Google Scholar] [CrossRef]
- Pinto, S.C.S.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, characterization and evaluation of a carbazolyl-BODIPY as a fluorimetric chemosensor for F−. Chem. Proc. 2022, 8, 20. [Google Scholar] [CrossRef]
- Kollmannsberger, M.; Rurack, K.; Resch-genger, U.; Daub, J. Ultrafast charge transfer in amino-substituted boron dipyrromethene dyes and its inhibition by cation complexation: A new design concept for highly sensitive fluorescent probes. Chem. Soc. 1998, 102, 10211–10220. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, C.; Li, J.; Wang, Z.; Wu, M.; Hao, E. β-formyl-BODIPYs from the Vilsmeier−Haack reaction. J. Org. Chem. 2009, 74, 7525–7528. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Demas, J.N.; Crosby, G.A. Measurement of photoluminescence quantum yields. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.C.R.; Boland, M.L.; Costa, S.P.G.; Raposo, M.M.M. Anion Dual Mode Fluoro-Chromogenic Chemosensor Based on a BODIPY Core. Eng. Proc. 2022, 27, 6. https://doi.org/10.3390/ecsa-9-13191
Gonçalves RCR, Boland ML, Costa SPG, Raposo MMM. Anion Dual Mode Fluoro-Chromogenic Chemosensor Based on a BODIPY Core. Engineering Proceedings. 2022; 27(1):6. https://doi.org/10.3390/ecsa-9-13191
Chicago/Turabian StyleGonçalves, Raquel C. R., Mathilde L. Boland, Susana P. G. Costa, and M. Manuela M. Raposo. 2022. "Anion Dual Mode Fluoro-Chromogenic Chemosensor Based on a BODIPY Core" Engineering Proceedings 27, no. 1: 6. https://doi.org/10.3390/ecsa-9-13191
APA StyleGonçalves, R. C. R., Boland, M. L., Costa, S. P. G., & Raposo, M. M. M. (2022). Anion Dual Mode Fluoro-Chromogenic Chemosensor Based on a BODIPY Core. Engineering Proceedings, 27(1), 6. https://doi.org/10.3390/ecsa-9-13191






