Abstract
In this research, a nanoporous platinum film is synthesized using the dealloying method. Platinum–copper (Pt–Cu) alloy films with a thickness of approximately 50 nm are prepared on glass using the magnetron-co-sputtering technique. In order to obtain nanoporous Pt, the Pt–Cu alloy films are dealloyed in 1 M nitric acid solution for different intervals. It is observed that when dealloyed in the nitric acid solution for 5 h, Cu was completely removed from the alloy, and nanoporous Pt with a regular pore structure was obtained. Then, the fabricated nanoporous Pt film is tested for hydrogen detection in the concentration range of 10 ppm and–5% hydrogen at various temperatures. The results demonstrated that the sensitivity of nanoporous Pt is about 6.5 for exposure to 1% hydrogen, and the sensing mechanism of nanoporous Pt could be explained by the surface scattering phenomenon.
1. Introduction
Hydrogen, an abundant element in the universe, is an efficient, clean, and renewable future energy source. Hydrogen is also an industrial gas used in many areas such as chemistry (as a reducing medium in crude oil refinement, plastics, the flat glass industry, etc.), semiconductors (as a process gas in thin-film deposition and annealing atmospheres), food products (for oil and fat hydrogenation), and transportation (in cars, trains, fuel cells, and spacecraft) [1]. However, it is a gas with a lower explosion limit of 4% in air and a low ignition energy. Therefore, a slight gas leak is a cause for serious concern, and the use of hydrogen poses a severe safety hazard. Hydrogen gas cannot be detected by the human senses, and it is tasteless, colorless, and odorless. So, when hydrogen or hydrogen-containing gases are used, safety is an important parameter. Therefore, the development of hydrogen sensors is vital, and these sensors should respond quickly, have a wide sensing range, and be capable of being deployed in city-scale networks [2]. In addition, a great deal of research is underway to continuously improve sensor parameters, such as selectivity, sensitivity, reliability, and response time, as well as diminish cost, sensor size, and power consumption, to meet future demands for the use of hydrogen in technology [3].
Metal and metal alloy nanomaterials, which have very good physical and chemical properties, have attracted great attention due to their important applications in various fields such as catalysis, batteries, actuators, optics, sensors, and medical therapy [4,5,6]. The performance of nanomaterials can be increased by adjusting parameters such as their size, shape, morphology, composition, and structure. Porous nanomaterials have superior physical properties, such as a very good geometric structure, large surface area, fine pores, and stability [7,8,9,10,11]. Dealloying, one of the porous material production methods, is a corrosion process wherein the selective dissolution of an active component from a homogeneous, single-phase alloy consisting of bi- or multi-component metals with different chemical activities under suitable corrosion conditions leaves a porous residue behind [12,13]. The network architectures of dealloyed metals contain nanoscale struts and ligaments. Such a structural feature enhances their mechanical performance for applications as functional or lightweight high-strength materials in sensing and actuating devices [14].
Platinum (Pt), which is one of the noble metals, allows these materials to be widely used in areas such as the chemical, petrochemical, pharmaceutical, electronic, and automotive industries thanks to its remarkable resistance to corrosion and outstanding catalytic and electrical properties [10,11]. Pt, Pd, and their alloys with other metals are used as sensing layers for resistive metallic hydrogen sensors [15]. They are also used as sensing layers for optical, magnetic, and mechanical hydrogen sensors, and as contact materials for semiconductor-resistive hydrogen sensors and work function-based hydrogen sensors. The absorption of molecular hydrogen by Pd or Pt—causing physical property changes such as electrical, mass, volume, and magnetic alterations—can be used to develop hydrogen sensors [16]. There are few studies about Pt-based resistive hydrogen sensors compared to Pd-based resisitive hydrogen sensors.
In this report, nanoporous Pt films are fabricated using the dealloying method. The temperature- and concentration-dependent hydrogen gas-sensing properties of these films are studied.
2. Materials and Methods
Platinum–copper (Pt–Cu) alloy films with an approximately 50 nm thickness were coated on a microscope glass slide by using the magnetron-co-sputtering method with an atomic ratio of 15:85. In order to obtain nanoporous Pt, Pt–Cu alloy films were dealloyed in 1 M nitric acid (HNO3) solution at different intervals (from 15 min to 20 h). NANOVAK 400 PVD system was used for all metallic film coatings and consists of two sputtering and two thermal evaporator sources. The schematic representation of nanoporous Pt production using the dealloying method is given in Figure 1a. Nanoporous Pt, which dealloyed for 5 h, was heat treated at different temperatures from 200 °C to 500 °C.
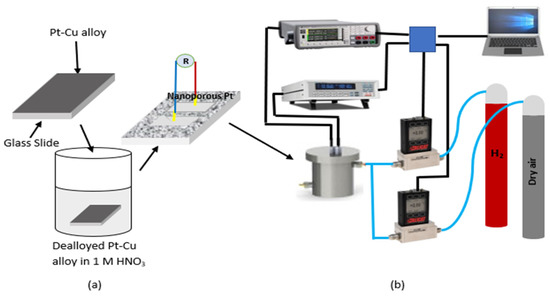
Figure 1.
Schematic diagrams of the fabrication of nanoporous Pt with dealloying method (a) and resistive gas-sensing measurement setup (b).
Scanning Electron Microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) that was directly incorporated into the SEM were used to clarify the morphologies and composition of nanoporous Pt and Pt–Cu alloy films, respectively. In order to determine hydrogen gas-sensing properties, two silver (Ag) electrodes were coated on the top of nanoporous Pt by using a thermal evaporator with a shadow mask to measure the two-point resistance. Figure 2 shows a schematic illustration for a resistive-type sensor device measurement setup. The resistance of nanoporous Pt was continuously recorded by utilizing a two point-probe with Keithley 2700 multimeter during changing of the atmosphere of a homemade measurement cell. The measurement cell was a flow-type aluminum chamber and included a sample holder capable of being heated. Two mass flow control units (Alicat) were used to control hydrogen gas concentration from 10 ppm to 5%. A schematic diagram of hydrogen gas sensor measurement system is given in Figure 1b. Lakeshore 335 temperature controller was used to change the temperature from 25 °C to 150 °C. All measurement data were recorded using LabVIEW program with a GPIB data acquisition system connected to a personal computer.
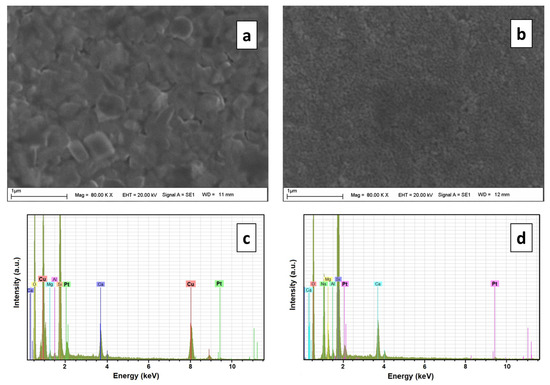
Figure 2.
SEM images of Pt–Cu alloy film (a) and nanoporous Pt film (b) that were fabricated with dealloying method in 1 M HNO3 for 5 h. EDX spectrums of Pt–Cu alloy film (c) and nanoporous Pt film (d).
3. Results and Discussion
Figure 2a shows an SEM image of the Pt–Cu alloys before dealloying and Figure 2b shows an SEM image of the Pt–Cu alloys after dealloying in 1 M HNO3 for 5 h. According to the SEM results, the films that de-alloyed for 5 h had a small-grained and smooth, porous structure. The EDX spectrum results of the Pt–Cu alloy before dealloying and nanoporous Pt etched in 1 M HNO3 solution for 5 h are given in Figure 2c,d, respectively. The peaks of elements such as Ca, Mg, Al, Si, O, Pt, and Cu were determined in the samples prepared according to the EDX results. The peaks of Ca, Mg, Al, and Si originate from the material in the structure of the microscope’s glass substrate. The EDX peaks of Cu and Pt come from the Pt–Cu alloy film that was coated on the glass, as seen in Figure 2c. When the two graphs are compared, it is clear that when the Pt–Cu alloy containing 85% Cu is de-alloyed in 1 M HNO3 solution for 5 h, Cu is completely removed from the structure. Since there is no peak of Cu in the EDX results given in Figure 2d, it was deduced that when de-alloyed in nitric acid solution for 5 h, Cu was utterly removed from the alloy, and nanoporous Pt with a regular pore structure was obtained.
Figure 3a shows the resistance change of the nanoporous Pt film during exposure to various hydrogen concentrations in the range of 10 ppm to 5% at the temperature of 150 °C. The resistance of nanoporous Pt film decreased when the measurement cell was purged with 10 ppm hydrogen; then, during the cleaning of the measurement cell with high-purity dry air, the resistance increased slowly. Similar behaviors were obtained for the remaining indicated hydrogen concentrations. The change in the resistance of nanoporous Pt enhances while the hydrogen concentration increases. The hydrogen-sensing mechanism can be explained as follows. During the resistance of the nanoporous Pt film’s measurement under dry air flow, the surface of the film is covered with adsorbed oxygen. While hydrogen is exposed to the film, hydrogen atoms dislocate with oxygen atoms, and the degree of electron surface scattering decreases. So, the decrease in the resistances of the film can be elucidated by the surface-scattering phenomenon. A similar behavior was reported for Pt nanowire [17] and Pt thin films [18,19,20]. Figure 3b shows sensitivity as a function of the logarithmic hydrogen concentration of the nanoporous Pt film at 150 °C, in which the sensitivity is increased as the concentration enhances. Figure 3c shows the sensitivity versus temperature curve of the nanoporous Pt exposed to 1% hydrogen. The sensitivity of nanoporous Pt film rises with the temperature, and this behavior could be related to the activation process.
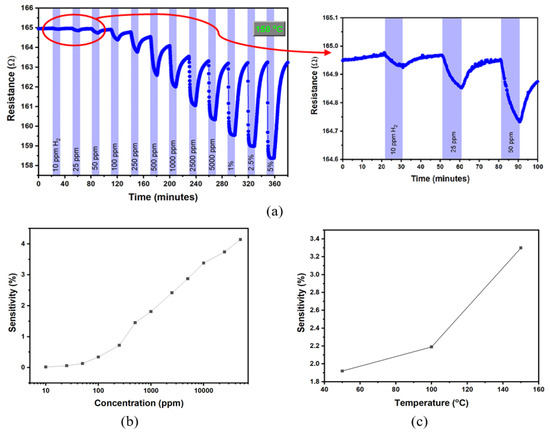
Figure 3.
The resistance versus time graph (a) and sensitivity as a function of concentration chart (b) of nanoporous Pt film exposed to broad hydrogen concentrations (10 ppm–5%) at the temperature of 150 °C. (c) The sensitivity versus temperature curve of nanoporous Pt exposed to 1% hydrogen.
4. Conclusions
A nanoporous Pt film was successfully fabricated by dealloying Pt–Cu alloy thin films that were coated on microscope glass substrates with the magnetron-co-sputtering method. The temperature- and hydrogen concentration-dependent gas-sensing properties of the nanoporous film were studied. This nanoporous Pt film could be used for low concentration hydrogen detection, such as in safety issues, hydrogen leak detection, and the detection of molecular hydrogen in exhaled breath for diagnosis.
Author Contributions
Conceptualization, M.S., A.A., R.Z. and N.K.; methodology, M.S., A.A., R.Z. and N.K.; software, N.K.; validation, M.S., A.A., R.Z. and N.K.; formal analysis, M.S., A.A., R.Z. and N.K.; investigation, M.S. and A.A.; resources, R.Z. and N.K.; data curation, M.S. and A.A.; writing—original draft preparation, M.S., A.A., R.Z. and N.K.; writing—review and editing, M.S., A.A., R.Z. and N.K.; visualization, M.S.; supervision, R.Z. and N.K.; project administration, R.Z. and N.K.; funding acquisition, R.Z. and N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) with a project number of 121M681.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fasaki, I.; Suchea, M.; Mousdis, G.; Kiriakidis, G.; Kompitsas, M. The effect of Au and Pt nanoclusters on the structural and hydrogen sensing properties of SnO2 thin films. Thin Solid Films 2009, 518, 1109–1113. [Google Scholar] [CrossRef]
- Rane, S.; Arbuj, S.; Rane, S.; Gosavi, S. Hydrogen sensing characteristics of Pt–SnO2 nano-structured composite thin films. J. Mater. Sci. Mater. Electron. 2015, 26, 3707–3716. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Martens, B.; Luo, Z.; Xu, D.; Wang, Y.; Zou, S.; Fang, J. Pt–Cu nanoctahedra: Synthesis and comparative study with nanocubes on their electrochemical catalytic performance. Chem. Sci. 2012, 3, 3302–3306. [Google Scholar] [CrossRef]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2012, 42, 2880–2904. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-X.; Lei, Z.-C.; Jiang, Z.-Y.; Hou, C.-P.; Liu, D.-Y.; Xu, M.-M.; Tian, Z.-Q.; Xie, Z.-X. Supersaturation-Dependent Surface Structure Evolution: From Ionic, Molecular to Metallic Micro/Nanocrystals. J. Am. Chem. Soc. 2013, 135, 9311–9314. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Kuroda, K. Rational Design of Mesoporous Metals and Related Nanomaterials by a Soft-Template Approach. Chem. Asian J. 2008, 3, 664–676. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef]
- Liang, J.; Du, X.; Gibson, C.; Du, X.W.; Qiao, S.Z. N-Doped Graphene Natively Grown on Hierarchical Ordered Porous Carbon for Enhanced Oxygen Reduction. Adv. Mater. 2013, 25, 6226–6231. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767. [Google Scholar] [CrossRef] [PubMed]
- Kloke, A.; Stetten, F.V.; Zengerle, R.; Kerzenmacher, S. Vertically oriented reduced graphene oxide supported dealloyed palladium–copper nanoparticles for methanol electrooxidation. Adv. Mater 2011, 23, 4976. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.V.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Kertis, F.; Snyder, J.; Govada, L.; Khurshid, S.; Chayen, N.; Erlebacher, J. Evolution of nanoporosity in dealloying. J. Manage 2010, 62, 50–56. [Google Scholar]
- Kunduraci, M. Dealloying technique in the synthesis of lithium-ion battery anode materials. J. Solid State Electrochem. 2016, 20, 2105–2111. [Google Scholar] [CrossRef]
- Kilinc, N. Resistive Hydrogen Sensors Based on Nanostructured Metals and Metal Alloys. Nanosci. Nanotechnol. Lett. 2013, 5, 825–841. [Google Scholar] [CrossRef]
- Kilinc, N.; Sanduvac, S.; Erkovan, M. Platinum-Nickel alloy thin films for low concentration hydrogen sensor application. J. Alloys Compd. 2021, 892, 162237. [Google Scholar] [CrossRef]
- Yang, F.; Donavan, K.C.; Kung, S.-C.; Penner, R.M. The Surface Scattering-Based Detection of Hydrogen in Air Using a Platinum Nanowire. Nano Lett. 2012, 12, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, N. Palladium and platinum thin films for low-concentration resistive hydrogen sensor: A comparative study. J. Mater. Sci. Mater. Electron. 2021, 32, 5567–5578. [Google Scholar] [CrossRef]
- Patel, S.V.; Gland, J.L.; Schwank, J.W. Film Structure and Conductometric Hydrogen-Gas-Sensing Characteristics of Ultrathin Platinum Films. Langmuir 1999, 15, 3307–3311. [Google Scholar] [CrossRef]
- Şennik, E.; Ürdem, Ş.; Erkovan, M.; Kılınç, N. Sputtered platinum thin films for resistive hydrogen sensor application. Mater. Lett. 2016, 177, 104–107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).